具有PD1启动子驱动IL-10的装甲人CAR - T细胞具有增强的抑制功能
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
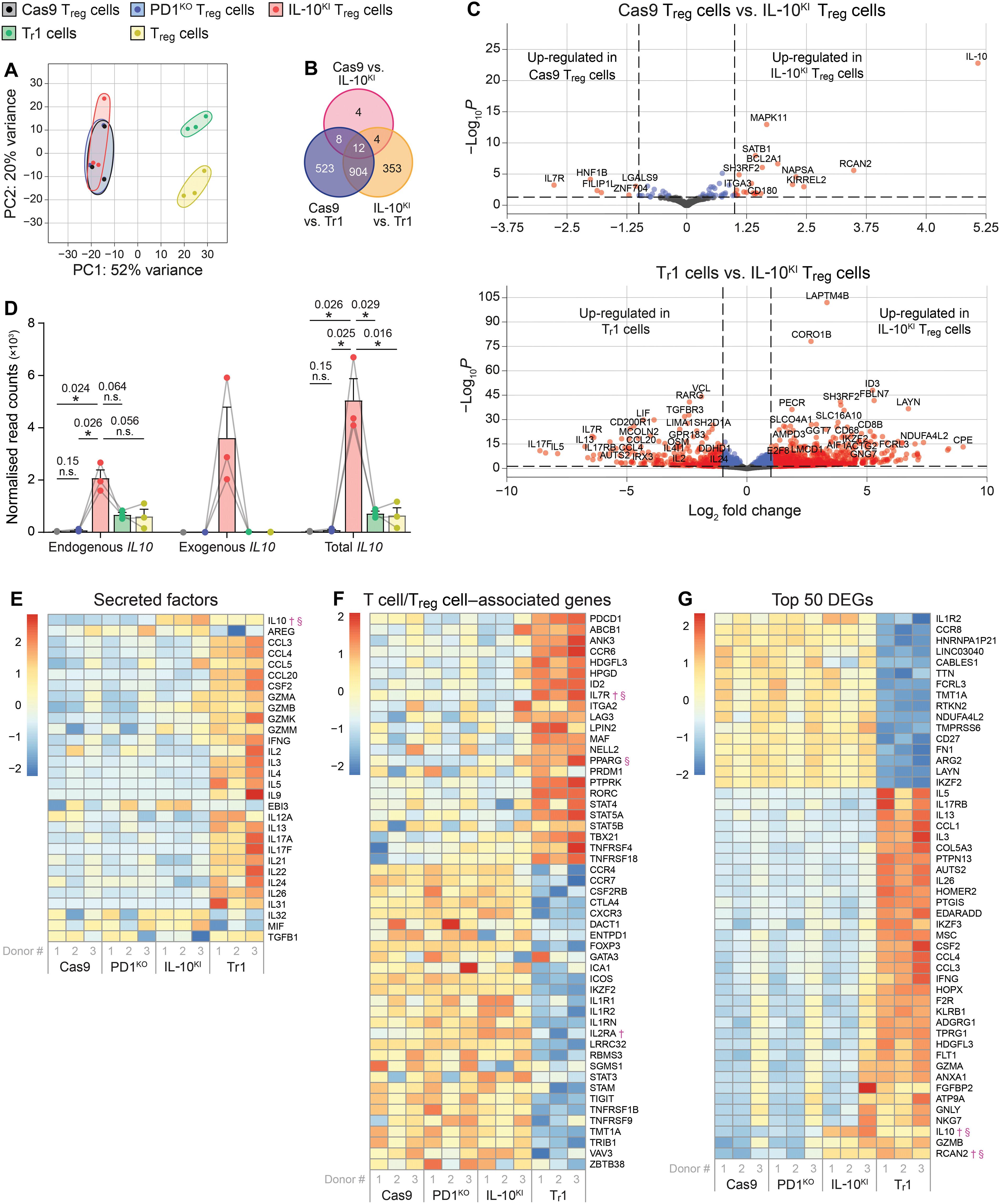
通过使用嵌合抗原受体(CARs),调节性T细胞(T reg细胞)疗法已经发生了转变。我们之前发现,与1型调节性T细胞(t1细胞)相比,人类T regg细胞产生IL-10的能力很低,并且控制先天免疫的能力有限。为了创建具有t1细胞样特性的“杂交”CAR- T regg细胞,我们研究了PDCD1位点是否可以被利用来赋予T regg细胞CAR调节的IL-10表达。crispr介导的PD1缺失增加了CAR- T细胞的活化,在PD1启动子的控制下敲入IL-10导致CAR诱导的IL-10分泌。通过增加树突状细胞和同种异体抗原特异性T细胞和胰岛自身抗原特异性T细胞的抑制,il - 10敲入改善了CAR - T细胞的功能。在体内,il - 10敲入的CAR - T reg细胞是稳定、安全的,并且可以抑制树突状细胞和异种移植物抗宿主病。crispr介导的工程同时去除抑制信号和增强抑制机制是一种以前未被探索的提高CAR - T细胞效力的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Armored human CAR Treg cells with PD1 promoter-driven IL-10 have enhanced suppressive function
Regulatory T cell (Treg cell) therapy has been transformed through the use of chimeric antigen receptors (CARs). We previously found that human Treg cells minimally produce IL-10 and have a limited capacity to control innate immunity compared to type 1 regulatory T cells (Tr1 cells). To create “hybrid” CAR Treg cells with Tr1 cell-like properties, we examined whether the PDCD1 locus could be exploited to endow Treg cells with CAR-regulated IL-10 expression. CRISPR-mediated PD1 deletion increased CAR Treg cell activation, and knock-in of IL10 under control of the PD1 promoter resulted in CAR-induced IL-10 secretion. IL10 knock-in improved CAR Treg cell function, as determined by increased suppression of dendritic cells and alloantigen- and islet autoantigen–specific T cells. In vivo, IL10 knock-in CAR Treg cells were stable, safe, and suppressed dendritic cells and xenogeneic graft-versus-host disease. CRISPR-mediated engineering to simultaneously remove an inhibitory signal and enhance a suppressive mechanism is a previously unexplored approach to improve CAR Treg cell potency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


