利用功能化腰果酚交联剂调整多硫化物的性能
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
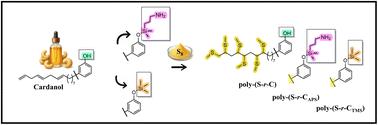
本研究介绍了单质硫与腰果酚及其硅烷功能化衍生物反硫化合成的表面功能化多硫化物的合成、表征和性能分析。制备了聚(S-r-C)、聚(S-r-CAPS)和聚(S-r-CTMS)三种聚硫化合物配方,研究了官能团对交联聚硫化合物性能的影响。腰果酚羟基与3-氨基丙基三乙氧基硅烷(APTES)或氯三甲基硅烷(TMS)反应可以修饰多硫化物网络。进行了表征,包括核磁共振光谱、红外光谱、扫描电镜、接触角测量、热重分析(TGA)、差示扫描量热法(DSC)和ASTM D3359交叉粘附测试。Poly-(S-r-C)和Poly-(S-r-CAPS)表现出与玻璃衬底的强附着力和更高的热稳定性,分别归因于氢键和Si-O-Si网络的形成。相比之下,含有非极性三甲基硅基的聚-(S-r-CTMS)表现出较弱的附着力和较低的热残留。结果强调了化学功能如何控制附着力,润湿性,溶解度和热行为。这项工作证明了可再生腰果酚基单体在设计可持续的富硫材料应用于涂料和粘合剂方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tuning the properties of polysulfides using functionalised cardanol crosslinkers†
This study presents the synthesis, characterisation, and property analysis of surface-functionalised polysulfides synthesised via inverse vulcanisation of elemental sulfur with cardanol and its silane-functionalised derivatives. Three polysulfide formulations, poly-(S-r-C), poly-(S-r-CAPS), and poly-(S-r-CTMS), were prepared to investigate the impact of functional groups on the crosslinked polysulfide's properties. Reacting the cardanol hydroxy group with either 3-aminopropyltriethoxysilane (APTES) or chlorotrimethylsilane (TMS) enabled the modification of the polysulfide networks. Characterisation, including NMR spectroscopy, FTIR spectroscopy, SEM, contact angle measurements, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and ASTM D3359 cross-hatch adhesion testing, was conducted. Poly-(S-r-C) and poly-(S-r-CAPS) demonstrated strong adhesion to glass substrates and higher thermal stability, attributed to hydrogen bonding and the formation of Si–O–Si networks, respectively. In contrast, poly-(S-r-CTMS), bearing nonpolar trimethylsilyl groups, exhibited weaker adhesion and lower thermal residue. The results highlight how chemical functionality governs adhesion, wettability, solubility, and thermal behavior. This work demonstrates the potential of renewable cardanol-based monomers in designing sustainable sulfur-rich materials for applications in coatings and adhesives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


