C, n -环亚胺偶极子和氧化剂在吡唑烷融合四氢喹啉和c3功能化喹啉合成中的双重作用
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以n -硅基烯胺和C, n -环亚甲基亚胺为原料,采用[3 + 2]环加成法,在不同的试剂配比下,选择性地合成了吡唑烷-熔融四氢喹啉和c3功能化喹啉。在本研究中,C, n -环亚甲基亚胺同时具有偶极子和氧化剂的双重作用,后者是前所未有的。通过逐步对照反应和氘标记研究,证实了C, n -环亚甲基亚胺的双重作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

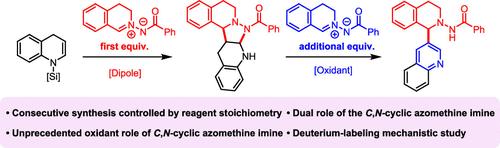
Dual Role of C,N-Cyclic Azomethine Imine as a Dipole and an Oxidant in the Synthesis of Pyrazolidine-Fused Tetrahydroquinolines and C3-Functionalized Quinolines
A selective synthesis of pyrazolidine-fused tetrahydroquinoline and C3-functionalized quinoline from N-silyl enamine and C,N-cyclic azomethine imine is presented, using [3 + 2] cycloaddition with varying reagent ratios. In this study, the C,N-cyclic azomethine imine serves dual roles as both dipole and oxidant, with the latter being unprecedented. The dual role of C,N-cyclic azomethine imine was confirmed through stepwise control reactions and deuterium-labeling studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


