桃金娘素D-E的仿生合成
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
从现成的生物遗传构建块中,仅用6-7个线性步骤,就实现了一种简洁高效的桃金娘共酮D-E的仿生合成。该合成的主要特点是锌介导的骨架重排反应,不需要稀有金属光催化剂和可见光。基于这种仿生合成,四种化合物(U2OS和143B)对骨肉瘤细胞表现出中等至优异的细胞毒活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
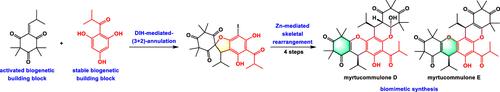
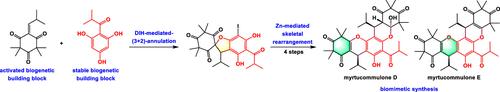
Biomimetic Synthesis of Myrtucommulones D–E
A concise and efficient biomimetic synthesis of myrtucommulones D–E has been achieved, proceeding in just 6–7 linear steps from readily available biogenetic building blocks. The key feature of the synthesis was the Zn-mediated skeletal rearrangement reaction, without the need for rare metal photocatalysts and visible light. Based on this biomimetic synthesis, four compounds demonstrated moderate to excellent cytotoxic activities against osteosarcoma cells (U2OS and 143B).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


