以硫代酸吡啶为供体-受体环丙烷的多米诺开环环化(DROC)合成功能化硫代吡喃衍生物
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
以硫代酸吡啶为原料,以Yb(OTf)3为刘易斯酸,K2CO3为碱,在温和的条件下,通过开环环反应(DROC),开发了一种制备5,6-二氢- 4h -硫代吡喃衍生物的有效途径。非外消旋da -环丙烷(ee 97%)与硫酸吡啶的sn2型对映反应可得到相应的非外消旋硫吡喃产品,产率高,对映特异性好(>97%)。这种方法为合成和生物学意义的硫吡喃框架提供了一种简单实用的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。

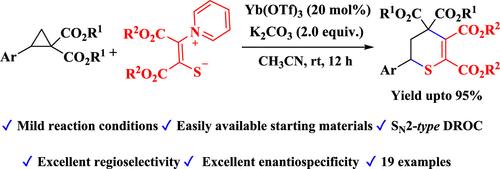
A Synthetic Route to Functionalized Thiopyran Derivatives via Domino Ring-Opening Cyclization (DROC) of Donor–Acceptor Cyclopropanes with Pyridinium Thiolates
An efficient route to 5,6-dihydro-4H-thiopyran derivatives is developed via domino ring-opening cyclization (DROC) of activated donor–acceptor (DA)-cyclopropanes with pyridinium thiolates in the presence of Yb(OTf)3 as the Lewis acid and K2CO3 as the base under mild conditions in moderate to excellent yields. Enantiospecific SN2-type DROC of nonracemic DA-cyclopropane (ee 97%) with pyridinium thiolate afforded the corresponding nonracemic thiopyran product with high yield and excellent enantiospecificity (>97%). This approach offers a straightforward and practical route to thiopyran frameworks of synthetic and biological significance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


