溶液化学对钒钛磁铁矿尾矿胶体稳定性和输运的影响机理
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
矿物风化和工业生产过程中产生的钒钛磁铁矿尾矿胶体由于其流动性和可释放性,会对地下水造成被忽视的钒污染。本研究通过实地调查、批量实验和柱状实验,研究了钒钛磁铁矿TCs在pH、离子强度(IS)、乙酸(AA)、草酸(OA)和柠檬酸(CA)等不同溶液化学条件下的稳定性和输运机制。结果表明,钒钛磁铁矿尾矿库地下水中存在TCs、AA、OA和CA。TCs之间的边-面相互作用促进了TCs在等电点周围的聚集。在所有pH和IS下,AA促进了TCs的聚集,而OA和CA抑制了TCs的聚集。在pH为7的条件下,添加10 mM AA、OA和CA后,TCs的临界凝血浓度(CCC)由34.83 mM增加到2.04、424.54和561.17 mM。随着低分子woas浓度的增加,诱导了更强的聚集。增加溶液pH和降低IS有利于TCs的迁移。在酸性和中性条件下,AA通过形成AA- tc -石英砂三元配合物抑制了TCs的转运,而OA和CA在所有ph下均促进了TCs的转运,酸浓度的变化对TCs的转运影响不显著。DLVO和XDLVO相互作用对TCs的稳定性和输运具有高度调控作用,采用改进的反应输运模型、Langmuirian动力学方程和成熟动力学模型可以很好地描述TCs的Langmuirian阻塞和成熟特征保留曲线。本研究首次系统研究了钒钛磁铁矿TCs在不同溶液化学条件下的行为,并结合能够分泌AA的植物为TCs的地下水污染防治提供了新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
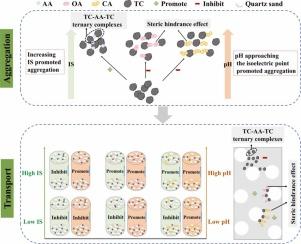
The influential mechanisms of solution chemistry on the stability and transport of vanadium-titanium magnetite tailing colloids
Vanadium-titanium magnetite tailing colloids (TCs) from mineral weathering and industrial production can cause overlooked vanadium groundwater pollution due to their mobility and releasable vanadium. This study investigated the stability and transport mechanisms of vanadium-titanium magnetite TCs under various solution chemistries, including pH, ionic strength (IS), acetic acid (AA), oxalic acid (OA), and citric acid (CA), via field survey, batch, and column experiments with numerical modeling. The results showed field-existing evidence of TCs, AA, OA, and CA in the groundwater of the V-Ti magnetite tailing pond. The edge-face interaction between TCs promoted TCs aggregation around the isoelectric point. AA promoted, while OA and CA inhibited TCs aggregation under all pH and IS. Stronger aggregation was induced by the increased concentration of AA, but contrary to OA and CA. The critical coagulation concentration (CCC) of TCs changed from 34.83 mM to 2.04, 424.54, and 561.17 mM after adding 10 mM AA, OA, and CA at pH 7. As the concentration of LMWOAs increased, stronger aggregation was induced. Increasing solution pH and decreasing IS promoted TCs transport. The ripening phenomenon occurred in the BTCs when IS exceeded 30 mM. AA inhibited TCs transport under acidic and neutral conditions by forming AA-TC-quartz sand ternary complexes, while OA and CA promoted transport at all pH. The variation of acid concentration showed insignificant effects on the TCs transport. DLVO and XDLVO interactions highly regulate TCs stability and transport, and the Langmuirian blocking and ripening featured retention profiles of TCs can be well described using the modified reactive transport model, the Langmuirian kinetic equation and the ripening kinetic model. This research is the first systematic study of vanadium-titanium magnetite TCs behaviors under different solution chemical conditions, and provided a novel strategy for groundwater pollution prevention of TCs by considering plants capable of secreting AA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


