肺炎克雷伯菌RamA、RarA和SoxS的适应性代偿性替加环素耐药机制研究
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-06-09
DOI:10.1016/j.ijantimicag.2025.107551
引用次数: 0
摘要
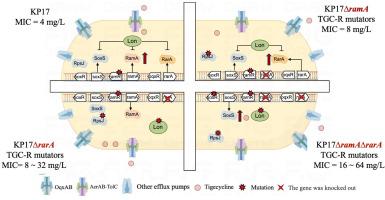
为了研究RamA、RarA和SoxS调节因子在肺炎克雷伯菌对替加环素耐药中的作用,我们在体外进化出单调控基因敲除或双调控基因敲除的碳青霉烯耐药(C248-ST11-blaKPC-2和C182-ST11-blaNDM-1)、粘菌素耐药(HK1-ST562-mcr-1)和替加环素耐药(KP17)菌株,以产生耐替加环素突变体。CRISPR-Cas9用于创建缺乏rarA、ramA、lon或其组合的KP17突变体。评估替加环素的突变预防浓度(MPC)和调控突变体的突变频率。通过生长曲线、体外竞争生长、血清杀菌活性、生物膜形成和过氧化氢抗性测试来评估突变体和亲本之间的表型差异。使用全基因组和RNA测序分析遗传和转录组变异。探讨RamA、RarA和SoxS在肺炎克雷伯菌替加环素耐药中的适应性代偿机制。碳青霉烯和粘菌素耐药菌株获得了高水平的替加环素耐药性,这导致了适应性成本和毒性降低。耐粘菌素菌株迅速进化出高水平的替加环素耐药性,最低抑制浓度可达256mg /L。RamRA-AcrAB/OqxAB途径是碳青霉烯类和粘菌素耐药菌株对替加环素耐药的关键。Lon与替加环素耐药性无关,但似乎与氧化应激有关。尽管敲除关键调控基因RamA和/或RarA不会阻碍替加环素耐药性的发展,但这些敲除会影响突变频率和MPCs,其中RarA敲除会增加突变位点的数量。RarA和SoxS在RamA缺失或双敲除时起补偿性调控作用。这些发现提高了我们对肺炎克雷伯菌对替加环素耐药机制的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Elucidating adaptive compensatory tigecycline resistance mechanisms of RamA, RarA and SoxS in Klebsiella pneumoniae
Background
The molecular regulation of tigecycline resistance is complex, and the roles of RamA, RarA, and SoxS regulons in tigecycline resistance are not fully understood.
Methods
Carbapenem-resistant (C248-ST11-blaKPC-2 and C182-ST11-blaNDM-1), colistin-resistant (HK1-ST562-mcr-1), and tigecycline-resistant (KP17) strains with single or double regulon gene knockouts were evolved in vitro to generate tigecycline-resistant mutants. CRISPR-Cas9 was used to create KP17 mutants lacking rarA, ramA, lon, or their combinations. The mutant prevention concentration (MPC) of tigecycline and mutation frequency of regulon mutants were assessed. Phenotypic differences between the mutants and parents were assessed using growth curves, in vitro competition growth, serum bactericidal activity, biofilm formation, and hydrogen peroxide resistance tests. Genetic and transcriptomic variations were analyzed using whole-genome and RNA sequencing.
Results
Acquired high-level tigecycline resistance in carbapenem-and colistin-resistant strains incurred fitness costs and reduced virulence. Colistin-resistant strains rapidly evolved high-level tigecycline resistance, with minimum inhibitory concentration of up to 256 mg/L. The RamRA-AcrAB/OqxAB pathway was pivotal for tigecycline resistance in carbapenem- and colistin resistant strains. Lon was not related to tigecycline resistance but appeared to be linked to oxidative stress. Although knocking out the key regulon genes RamA and/or RarA did not impede tigecycline resistance development, these knockouts influenced mutation frequencies and MPCs, with RarA knockout increasing the number of mutation sites.
Conclusion
RarA and SoxS served as compensatory regulons in the absence of RamA, or in double knockouts. These findings improved our understanding of the mechanisms underlying tigecycline resistance in K. pneumoniae.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


