光化学驱动钌催化吲哚与醇的区域选择性C-H功能化以获得吲哚-3-乙醛和双(吲哚基)甲烷。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了一种利用钌催化剂在吲哚和醇之间进行的光化学C-H功能化反应,以获得吲哚-3-乙醛和吲哚基甲烷支架。优化研究表明,钌催化剂结构的调整使吲哚-3-乙醛的收率较高,而直接使用RuCl3·3H2O则使吲哚基甲烷的收率较高。这些反应在操作简单的绿色条件下进行,提供了广泛的底物范围。本文章由计算机程序翻译,如有差异,请以英文原文为准。
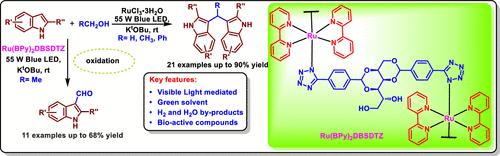
Photochemically Driven Ruthenium-Catalyzed Regioselective C–H Functionalization of Indoles with Alcohols to Access Indole-3-carbaldehyde and Bis(indolyl)methanes
We herein report a photochemical C–H functionalization reaction between indoles and alcohols to access indole-3-carbaldehyde and bis(indolyl)methane scaffolds using a ruthenium catalyst. Optimization studies revealed that the tuning in the ruthenium catalyst structure rendered indole-3-carbaldehyde in good yields, whereas the direct use of RuCl3·3H2O delivered bis(indolyl)methanes in good to excellent yields. These reactions proceed under operationally simple green conditions, offering a broad substrate scope.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


