CHO细胞表达重组蛋白的固定化金属亲和层析优化
IF 3.2
引用次数: 0
摘要
固定化金属亲和色谱法(IMAC)广泛应用于研究实验室,通过直接捕获和适度去除过程相关杂质,如宿主细胞蛋白(HCPs)和DNA,来纯化大肠杆菌(E. coli)中表达的his标记蛋白。然而,由于细胞培养基与IMAC树脂的不相容性问题,其在纯化哺乳动物细胞分泌的重组蛋白方面的应用受到限制。在这项研究中,我们使用Ni Sepharose excel树脂纯化了一个cho表达和分泌的带有His-tag的重组蛋白,该蛋白对EDTA和还原剂具有抗性,并优化了装载、洗涤和洗脱条件,以最大限度地提高蛋白质回收率和HCP清除率。树脂的最大负载能力为10 mg/mL。结果表明,结合Triton X-100、PS80和PS80;洗涤缓冲液中的TnBP或2-丙醇可以显著提高HCP的去除率,而在洗脱缓冲液中保持低盐浓度可以提高收率和产品质量。在最佳条件下进行的树脂寿命研究显示出可接受的收率,稳定的产品质量属性(PQAs),并且在54个净化循环中有效清洁。该协议提供了一个强大的IMAC净化策略,潜在地扩大了其工业应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
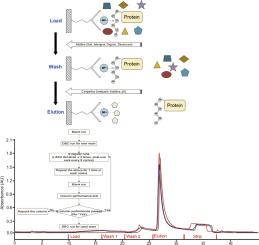
Optimization of immobilized metal affinity chromatography for a recombinant protein expressed in CHO cells
Immobilized metal affinity chromatography (IMAC) is widely used in research laboratories to purify His-tagged proteins expressed in Escherichia coli (E. coli) by enabling direct capture and moderate removal of process-related impurities, such as host cell proteins (HCPs) and DNA. However, its application for purifying recombinant proteins secreted by mammalian cells is limited due to incompatibility issues between cell culture media and IMAC resins. In this study, we purified a CHO-expressed and secreted recombinant protein with His-tag using a Ni Sepharose excel resin, resistant to EDTA and reducing agents, and optimized loading, washing, and elution conditions to maximize protein recovery and HCP clearance. The resin demonstrated a maximum load capacity of 10 mg/mL. Results show that incorporating Triton X-100, PS80 & TnBP, or 2-propanol in washing buffers significantly improves HCP removal while maintaining low salt concentrations in the elution buffer enhances both yield and product quality. Resin lifetime studies conducted under optimal conditions showed acceptable yields, stable product quality attributes (PQAs), and effective cleaning over 54 purification cycles. This protocol provides a robust IMAC purification strategy, potentially broadening its industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of chromatography open
Analytical Chemistry
CiteScore
2.50
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


