利用腈基导向基团对苯酚衍生物进行超选择性碳氢功能化
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-06-03
DOI:10.1021/acs.joc.5c0081210.1021/acs.joc.5c00812
引用次数: 0
摘要
虽然邻羟基活化已经得到了很好的探索,但由于区域选择性的限制,苯酚的间羟基功能化仍然具有挑战性。在这里,我们介绍了在温和的室温条件下使用脂肪族腈模板的位置选择性间碳-氢烯烃策略。该方法利用丁腈-金属相互作用来实现高效的碳氢活化,克服了传统方法所需的恶劣条件。该方法底物范围广,效率高,已成功应用于类药物和天然产物衍生物,收率高,选择性好。本文章由计算机程序翻译,如有差异,请以英文原文为准。
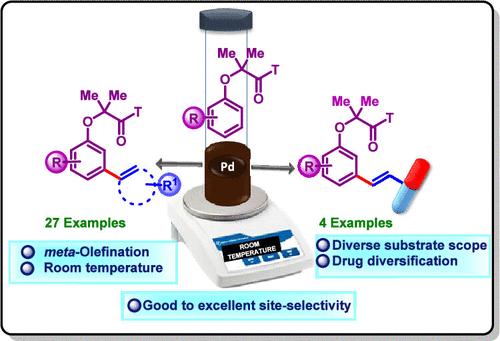
The meta-Selective C–H Functionalization of Phenol Derivatives Using a Nitrile-Based Directing Group
While ortho-C–H activation has been well explored, meta-C–H functionalization of phenols remains challenging due to regioselectivity constraints. Here, we introduce a site-selective meta-C–H olefination strategy using an aliphatic nitrile template under mild room-temperature conditions. This approach leverages nitrile–metal interactions to enable efficient C–H activation, overcoming the harsh conditions required in traditional methods. With a broad substrate scope and high efficiency, this method has been successfully applied to drug-like and natural product derivatives, delivering high yields and selectivities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


