胸腺模拟细胞的发育轨迹和进化起源
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
T细胞自我耐受谱的产生依赖于胸腺上皮中外周自身抗原的表达1和一小群细胞的存在,这些细胞模仿外周组织的不同表型2,3,4,5,6,7。虽然自体抗原表达的分子基础已被广泛研究,但胸腺模拟细胞的发育起源和分化途径仍有待确定。此外,在许多脊椎动物胸腺微环境中,肌样细胞和其他外周细胞类型的组织学鉴定对这种独特耐受机制的进化起源提出了疑问。在小鼠发育过程中,模拟细胞在微环境中出现两个连续的波。表现出肌肉细胞、离子细胞、杯状细胞和纤毛细胞转录特征的细胞在出生前就出现了,而其他细胞,如那些模仿肠肝细胞和皮肤角化细胞的细胞,则在出生后出现。这两组对由Foxn1和Ascl1缺失、转录因子Foxn1的亚型表达以及信号分子BMP4和FGF7的过表达引起的胸腺上皮细胞祖细胞池的调节也有不同的反应。在用进化上古老的Foxn1/4基因家族成员(包括头脊索文文鱼的Foxn4基因和软骨鱼的Foxn4和Foxn1基因)替代小鼠Foxn1重建的胸腺微环境中,也观察到模拟细胞群体的差异。有些细胞类型,如纤毛细胞,在胸腺发育时缺乏FOXN1,而后天出现的模拟细胞,如肠肝细胞,则需要脊椎动物特异性转录因子FOXN1的活性。软骨鱼类的胸腺和七鳃鳗的胸腺样体(一种无颌脊椎动物的代表)表现出另一种适应性免疫系统,它们也含有表达编码外周组织成分的基因的细胞,如肝脏特异性蛋白质转甲状腺素。我们的研究结果提示了胸腺上皮遗传网络连续变化的进化模型,使外周抗原表达和模拟细胞形成的协调贡献能够实现对脊椎动物特异性组织(如肝脏)的中枢耐受11,12。本文章由计算机程序翻译,如有差异,请以英文原文为准。

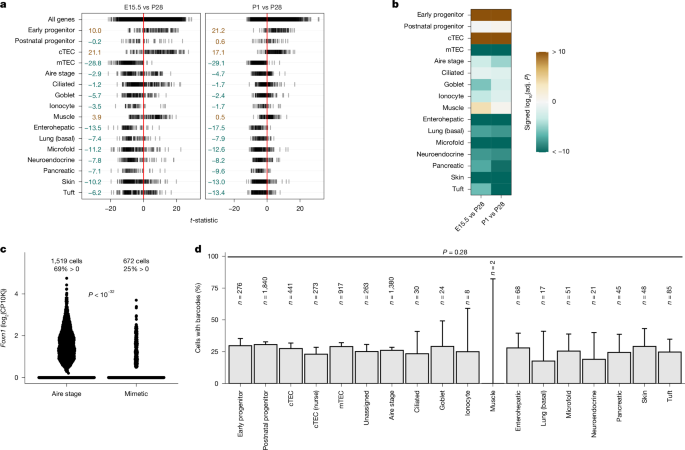
Developmental trajectory and evolutionary origin of thymic mimetic cells
The generation of self-tolerant repertoires of T cells depends on the expression of peripheral self antigens in the thymic epithelium1 and the presence of small populations of cells that mimic the diverse phenotypes of peripheral tissues2–7. Whereas the molecular underpinnings of self-antigen expression have been extensively studied8, the developmental origins and differentiation pathways of thymic mimetic cells remain to be identified. Moreover, the histological identification of myoid and other peripheral cell types as components of the thymic microenvironment of many vertebrate species9 raises questions regarding the evolutionary origin of this unique tolerance mechanism. Here we show that during mouse development, mimetic cells appear in the microenvironment in two successive waves. Cells that exhibit transcriptional signatures characteristic of muscle, ionocyte, goblet and ciliated cells emerge before birth, whereas others, such as those that mimic enterohepatic cells and skin keratinocytes, appear postnatally. These two groups also respond differently to modulations of thymic epithelial cell progenitor pools caused by deletions of Foxn1 and Ascl1, expression of a hypomorphic variant of the transcription factor FOXN1, and overexpression of the signalling molecules BMP4 and FGF7. Differences in mimetic cell populations were also observed in thymic microenvironments reconstructed by replacement of mouse Foxn1 with evolutionarily ancient Foxn1/4 gene family members, including the Foxn4 gene of the cephalochordate amphioxus and the Foxn4 and Foxn1 genes of a cartilaginous fish. Whereas some cell types, such as ciliated cells, develop in the thymus in the absence of FOXN1, mimetic cells that appear postnatally, such as enterohepatic cells, require the activity of the vertebrate-specific transcription factor FOXN1. The thymus of cartilaginous fishes and the thymoid of lampreys, a representative of jawless vertebrates, which exhibit an alternative adaptive immune system10, also harbour cells that express genes encoding peripheral tissue components such as the liver-specific protein transthyretin. Our findings suggest an evolutionary model of successive changes of thymic epithelial genetic networks enabling the coordinated contribution of peripheral antigen expression and mimetic cell formation to achieve central tolerance for vertebrate-specific innovations of tissues such as the liver11,12. In mice, tissue-mimetic cells appear in thymus in two distinct waves, creating two distinct pools of mimetic cells, each expressing antigens characteristic of different cell types.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


