疟疾感染红细胞上显示的RIFINs结合KIR2DL1和KIR2DS1
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
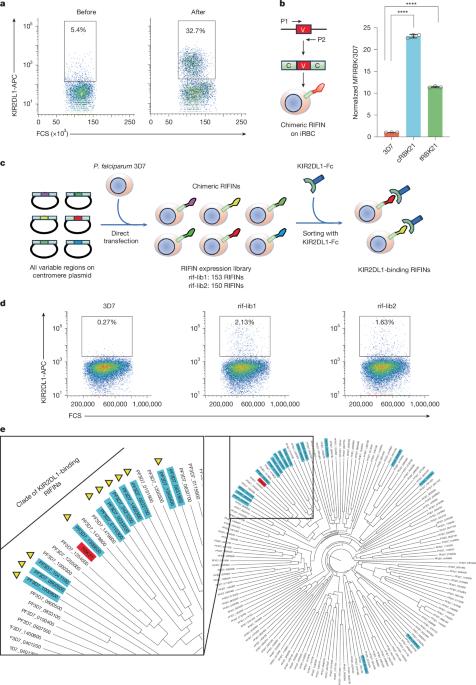
自然杀伤(NK)细胞使用抑制和激活免疫受体来区分人类细胞和病原体。这些受体发出的信号决定NK细胞是否被激活并破坏靶细胞。在某些情况下,如杀伤性免疫球蛋白样受体,免疫受体是成对的,抑制性和激活性受体含有几乎相同的细胞外配体结合域,与不同的细胞内信号域偶联1。先前的研究表明,在恶性疟原虫感染的红细胞表面显示的重复穿插家族(RIFIN)蛋白可以与抑制性免疫受体结合,抑制NK细胞的激活2,3,减少寄生虫的杀伤。然而,没有病原体衍生的配体被确定为任何人类激活受体。在这里,我们发现了一个RIFINs分支,它与抑制性免疫受体KIR2DL1的结合比KIR2DL1与人类配体(MHC I类)的结合更强。这种相互作用介导抑制性信号传导并抑制表达kir2dl1的NK细胞的激活。我们发现KIR2DL1结合的RIFINs在非洲和亚洲的野外分离菌株中都很丰富,并揭示了这两种RIFINs如何与KIR2DL1结合。KIR2DL1的RIFIN结合面在同源激活免疫受体KIR2DS1中是保守的。我们发现结合kir2dl1的RIFINs也可以结合KIR2DS1,从而激活表达KIR2DS1的NK细胞。本研究表明,激活杀伤性免疫球蛋白样受体可以招募NK细胞靶向病原体,并揭示了激活免疫受体在控制疟疾感染中的潜在作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
RIFINs displayed on malaria-infected erythrocytes bind KIR2DL1 and KIR2DS1
Natural killer (NK) cells use inhibitory and activating immune receptors to differentiate between human cells and pathogens. Signalling by these receptors determines whether an NK cell becomes activated and destroys a target cell. In some cases, such as killer immunoglobulin-like receptors, immune receptors are found in pairs, with inhibitory and activating receptors containing nearly identical extracellular ligand-binding domains coupled to different intracellular signalling domains1. Previous studies showed that repetitive interspersed family (RIFIN) proteins, displayed on the surfaces of Plasmodium falciparum-infected erythrocytes, can bind to inhibitory immune receptors and dampen NK cell activation2,3, reducing parasite killing. However, no pathogen-derived ligand has been identified for any human activating receptor. Here we identified a clade of RIFINs that bind to inhibitory immune receptor KIR2DL1 more strongly than KIR2DL1 binds to the human ligand (MHC class I). This interaction mediates inhibitory signalling and suppresses the activation of KIR2DL1-expressing NK cells. We show that KIR2DL1-binding RIFINs are abundant in field-isolated strains from both Africa and Asia and reveal how the two RIFINs bind to KIR2DL1. The RIFIN binding surface of KIR2DL1 is conserved in the cognate activating immune receptor KIR2DS1. We find that KIR2DL1-binding RIFINs can also bind to KIR2DS1, resulting in the activation of KIR2DS1-expressing NK cells. This study demonstrates that activating killer immunoglobulin-like receptors can recruit NK cells to target a pathogen and reveals a potential role for activating immune receptors in controlling malaria infection. Certain RIFINs from Plasmodium falciparum can bind to both inhibitory (KIR2DL1) and activating (KIR2DS1) immune receptors on natural killer cells, demonstrating the potential role of activating killer immunoglobulin-like receptors in targeting pathogens and controlling malaria infection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


