金属掺杂石墨氮化碳非共价键辅助MCN催化二氧化碳的研究
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
随着碳中和需求的提高,开发高效的催化材料将大气中的二氧化碳有效地转化为增值化学品至关重要。研究较多的MNxC单原子催化剂通常发生单中心CO2还原反应(CO2RR)。在本研究中,通过在石墨氮化碳中掺杂Sc/Y/La/Ac来设计MCN催化剂,期望通过金属中心和邻近的非金属配体原子来制造多中心协同催化。利用相对论密度泛函理论计算结合分子动力学模拟对CO2RR进行了系统的探索。所有这些催化剂都通过弯曲二氧化碳的刚性线性结构来激活二氧化碳。CO2→M负性键只出现在Sc的加合物中,而在Y、La和Ac的CO2加合物中还发现了2个非共价键。催化剂产生了3个催化活性位点(MCN),它们与CO2的CO形成一个平面五元环。从热力学角度来看,YCN配合物具有最佳的催化潜力。对CO2吸附和CO脱附的自由能变化分别为负(-0.247 eV)和中等(0.662 eV)。进一步的几何/电子结构和化学键分析证实了MCN催化剂在活化CO2方面的优异稳定性和电子转移能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
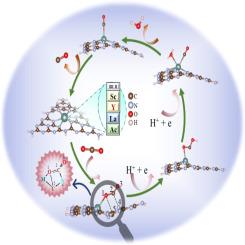
Noncovalent bond-aided MCN catalysis towards carbon dioxide by metal-doped graphitic carbon nitride
With raising carbon neutrality needs, it is crucial to develop efficient catalytic materials that effectively convert atmospheric CO2 to value-added chemicals. The well-studied M![]() Nx
Nx![]() C single-atom catalysts usually experienced a single-center CO2 reduction reaction (CO2RR). In the work, the M
C single-atom catalysts usually experienced a single-center CO2 reduction reaction (CO2RR). In the work, the M![]() C
C![]() N catalysts have been designed by doping Sc/Y/La/Ac into graphitic carbon nitride, in anticipation of fabricating multi-center synergistic catalysis from not only the metal center but adjacent non-metal ligand atoms as well. The CO2RR has been systematically explored using relativistic density functional theory calculations in combination with molecular dynamics simulations. All these catalysts activated CO2 by bending its rigid linear structure. The CO2→M dative bond appears only in the adduct of Sc. Unexpectedly, additional two non-covalent tetrel bonds were recognized in the CO2 adducts of Y, La and Ac. Three catalytic active sites (M
N catalysts have been designed by doping Sc/Y/La/Ac into graphitic carbon nitride, in anticipation of fabricating multi-center synergistic catalysis from not only the metal center but adjacent non-metal ligand atoms as well. The CO2RR has been systematically explored using relativistic density functional theory calculations in combination with molecular dynamics simulations. All these catalysts activated CO2 by bending its rigid linear structure. The CO2→M dative bond appears only in the adduct of Sc. Unexpectedly, additional two non-covalent tetrel bonds were recognized in the CO2 adducts of Y, La and Ac. Three catalytic active sites (M![]() C
C![]() N) are rendered by catalysts, which form a planar five-membered ring with C
N) are rendered by catalysts, which form a planar five-membered ring with C![]() O of CO2. From a thermodynamic perspective, the Y
O of CO2. From a thermodynamic perspective, the Y![]() C
C![]() N complex suggests the best catalytic potential. It has the most negative (-0.247 eV) and moderate (0.662 eV) free energy changes for CO2 adsorption and CO desorption, respectively. Further analyses on geometric/electronic structures and chemical bonding confirm the excellent stability and electronic transfer capability of M
N complex suggests the best catalytic potential. It has the most negative (-0.247 eV) and moderate (0.662 eV) free energy changes for CO2 adsorption and CO desorption, respectively. Further analyses on geometric/electronic structures and chemical bonding confirm the excellent stability and electronic transfer capability of M![]() C
C![]() N catalyst in activating CO2.
N catalyst in activating CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


