新型Z-scheme Ni-MOF/g-C3N4异质结上铀的光催化还原
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
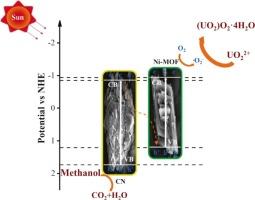
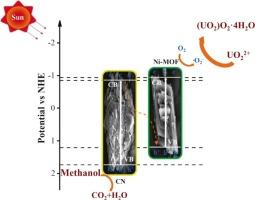
通过构建异质结策略增强了石墨相氮化碳(g-C3N4)。采用溶剂热法合成了CN/Ni-MOF复合材料,并对光还原铀的催化性能进行了研究。光催化还原实验表明,光照20 min后,最佳CN/Ni-MOF(300)的还原效率为94.24 %,速率常数为0.0877 min−1。值得注意的是,在不同催化剂用量、pH值和浓度的铀溶液中,铀的光催化还原表现出良好的活性。经过5次循环实验,还原效率仍保持在93.02 %。表征结果表明,参与反应的主要反应物质为e-和∙O2 -,最终反应产物为(UO2)O2·4H2O。对催化剂带隙结构的分析有助于对电子转移机理的研究,从而提出了一个合理的反应机理。当CN与Ni-MOF结合时,e-可能从CN向Ni-MOF迁移,加速了接触界面的迁移,从而增强了复合催化剂的光催化性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photocatalytic reduction of uranium over a novel Z-scheme Ni-MOF/g-C3N4 heterojunction
The graphite phase carbon nitride (g-C3N4) was enhanced by constructing a heterojunction strategy. CN/Ni-MOF composites were synthesized using a solvothermal method, and the catalytic performance of photoreduced uranium were investigated. The photocatalytic reduction experiment demonstrated the reduction efficiency of the optimal CN/Ni-MOF(300) was 94.24 % and the rate constant was 0.0877 min−1 after 20 min illumination. Notably, the photocatalytic reduction of uranium exhibited good activity in various catalyst dosages, pH, and concentrations of uranium solution. Furthermore, after 5 cycles of experiments, the reduction efficiency of 93.02 % was still sustained. The characterization results indicated that the main reactive substances involved in the reaction were e- and ∙O2–, and the final reaction product was (UO2)O2·4H2O. An analysis of the bandgap structure of the catalyst facilitated the examination of the electron transfer mechanism, resulting in the proposal of a plausible reaction mechanism. When CN is integrated with Ni-MOF, e- may migrate from CN to Ni-MOF, accelerating the migration at the contact interphase, thereby enhancing the photocatalytic performance of the composite catalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


