假性伯克霍尔德菌耐药和泛敏感菌株的全基因组测序分析揭示了新的耐药生物标志物。
IF 2.6
4区 医学
Q3 INFECTIOUS DISEASES
引用次数: 0
摘要
由革兰氏阴性杆菌假马氏伯克霍尔德菌(Bp)引起的类meliosis,由于其潜在的耐药性,可能严重限制现有的治疗选择,对健康构成重大威胁。为此,我们对38株耐药(DR) Bp和300株药敏(DS) Bp进行了比较基因组分析,以确定与抗生素耐药性相关的遗传标记。我们的研究发现了7个与耐药性相关的重要单核苷酸多态性(snp): 2个与头孢他啶(CAZ)有关,5个与美罗培南(MEM)有关。通路分析显示,AMC耐药与脂肪酸代谢的改变有关,而CAZ耐药与膜蛋白通路的改变有关。这些发现强调了Bp如何通过各种机制对关键抗生素产生耐药性。此外,我们在已知的耐药基因中发现了21个新的遗传变异,包括15个snp和6个短插入或缺失(indels)。这些以前未报道的变异可能有助于产生耐药性,突出了Bp的遗传多样性和对抗菌压力的适应性。这些发现加深了我们对Bp耐药性的理解,并为提高诊断精度的遗传标记提供了有价值的见解。通过丰富耐药数据库,本工作为早期耐药预测提供了前瞻性工具,促进了及时有效的治疗策略。此外,它强调了遗传调查在解决类鼻疽病抗生素耐药性挑战中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
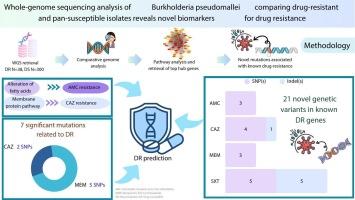
Whole-genome sequencing analysis of Burkholderia pseudomallei comparing drug-resistant and pan-susceptible isolates reveals novel biomarkers for drug resistance
Melioidosis, caused by the gram-negative bacterium Burkholderia pseudomallei (Bp), poses a significant health threat due to its potential for drug resistance, which can severely limit available treatment options. To investigate this, we conducted a comparative genomic analysis of 38 drug-resistant (DR) and 300 drug-susceptible (DS) Bp isolates to identify genetic markers associated with antimicrobial resistance. Our study identified seven significant single-nucleotide polymorphisms (SNPs) linked to drug resistance: two with ceftazidime (CAZ), and five with meropenem (MEM). Pathway analysis revealed that AMC resistance was associated with alterations in fatty-acid metabolism, whereas CAZ resistance was associated with changes in membrane protein pathways. These findings highlighted how Bp develops resistance to key antibiotics through various mechanisms. In addition, we discovered 21 novel genetic variants in known drug-resistance genes, including 15 SNPs and six short insertions or deletions (indels). These previously unreported variants could contribute to resistance, highlighting the genetic diversity and adaptability to antimicrobial pressures of Bp. These findings deepen our understanding of Bp drug resistance and offer valuable insights into genetic markers with the potential to enhance diagnostic precision. By enriching the resistance database, this work provides prospective tools for early resistance prediction, facilitating prompt and effective treatment strategies. Furthermore, it emphasizes the critical role of genetic investigations in addressing the challenge of antibiotic resistance in melioidosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Infection Genetics and Evolution
医学-传染病学
CiteScore
8.40
自引率
0.00%
发文量
215
审稿时长
82 days
期刊介绍:
(aka Journal of Molecular Epidemiology and Evolutionary Genetics of Infectious Diseases -- MEEGID)
Infectious diseases constitute one of the main challenges to medical science in the coming century. The impressive development of molecular megatechnologies and of bioinformatics have greatly increased our knowledge of the evolution, transmission and pathogenicity of infectious diseases. Research has shown that host susceptibility to many infectious diseases has a genetic basis. Furthermore, much is now known on the molecular epidemiology, evolution and virulence of pathogenic agents, as well as their resistance to drugs, vaccines, and antibiotics. Equally, research on the genetics of disease vectors has greatly improved our understanding of their systematics, has increased our capacity to identify target populations for control or intervention, and has provided detailed information on the mechanisms of insecticide resistance.
However, the genetics and evolutionary biology of hosts, pathogens and vectors have tended to develop as three separate fields of research. This artificial compartmentalisation is of concern due to our growing appreciation of the strong co-evolutionary interactions among hosts, pathogens and vectors.
Infection, Genetics and Evolution and its companion congress [MEEGID](http://www.meegidconference.com/) (for Molecular Epidemiology and Evolutionary Genetics of Infectious Diseases) are the main forum acting for the cross-fertilization between evolutionary science and biomedical research on infectious diseases.
Infection, Genetics and Evolution is the only journal that welcomes articles dealing with the genetics and evolutionary biology of hosts, pathogens and vectors, and coevolution processes among them in relation to infection and disease manifestation. All infectious models enter the scope of the journal, including pathogens of humans, animals and plants, either parasites, fungi, bacteria, viruses or prions. The journal welcomes articles dealing with genetics, population genetics, genomics, postgenomics, gene expression, evolutionary biology, population dynamics, mathematical modeling and bioinformatics. We also provide many author benefits, such as free PDFs, a liberal copyright policy, special discounts on Elsevier publications and much more. Please click here for more information on our author services .
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


