氮和硝酸盐电催化还原制氨的研究进展
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-02-16
DOI:10.1016/j.jiec.2025.02.029
引用次数: 0
摘要
在各种制氨策略中,氮的电催化还原是一种很有前途的方法,各种异相电催化剂正在被开发以促进氮还原反应(NRR)合成氨。然而,由于N2键的高稳定性和非极性性质,这些方法不适合大规模商业化,这进一步受到竞争析氢反应的挑战。升高的温度提高了法拉第效率(FE),但也增加了逆反应速率,导致氨解离。因此,由于硝酸盐具有更高的溶解度和更可行的键解,因此被用作电催化合成氨的替代试剂。电催化硝酸还原获得了较高的FE和NH3产率,超过了NRR中竞争性析氢反应。各种电催化剂已被用于有效的硝酸盐还原,包括金属氧化物基、金属合金、非氧化物、金属磷化物、金属硫化物、金属碳化物和碳基电催化剂。以前,研究人员指出了这些方法的优点,但缺乏比较评估。这篇综述解决了这一空白,深入研究了NH3电催化合成的机制,包括各种硝酸盐和NRR途径,并提出了一种可靠的氨检测方案。这项工作为创新电催化剂的最新进展提供了重要的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
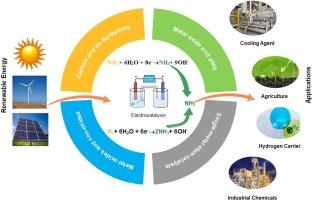
Critical review on electrocatalytic reduction of nitrogen and nitrate to ammonia
Among the various strategies for ammonia production, the electrocatalytic reduction of nitrogen is promising method and various heterogeneous electrocatalysts are being developed to facilitate the nitrogen reduction reaction (NRR) for ammonia synthesis. However, these methods are not suitable for large-scale commercialization due to the high stability and nonpolar nature of the N2 bond, which is further challenged by competing hydrogen evolution reactions. Elevated temperatures enhance the Faradaic efficiency (FE) but also increase the reverse reaction rate, leading to the dissociation of ammonia. Thus, nitrate has been used as an alternative reagent for electrocatalytic ammonia synthesis because it shows higher solubility and more feasible bond dissociation. Electrocatalytic nitrate reduction achieves high FE and NH3 yields, surpassing those of the competitive hydrogen evolution reactions experienced in the NRR. Various electrocatalysts have been used for effective nitrate reduction, including metal oxide-based, metal alloys, non-oxide, metal phosphides, metal sulfides, metal carbides, and carbon-based electrocatalysts. Previously, researchers addressed these methods’ advantages but lacked a comparative assessment. This review addresses this gap, thoroughly examines the mechanisms involved in the electrocatalytic synthesis of NH3, including various nitrate and NRR pathways, and proposes a reliable protocol for detecting ammonia. This work provides important insights on recent advancements in innovative electrocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


