长期暴露于环境浓度的恩诺沙星通过HPT轴破坏和造血失调损害斑马鱼中性粒细胞功能
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
氟喹诺酮类抗生素如恩诺沙星(ENR)在水生生态系统中的广泛检测引起了对生态和免疫影响的重大关注。虽然有急性ENR毒性记录,但慢性环境相关暴露的免疫毒性作用仍然知之甚少。本研究探讨了长期暴露于ENR (10-100 μg/L,浓度反映了水产养殖设施附近淡水系统的常见污染水平和废水处理失败导致的极端污染情况)影响斑马鱼免疫力的机制,重点是中性粒细胞功能障碍。我们的研究结果表明,ENR可使造血器官(血液、肾脏、脾脏)中的中性粒细胞计数减少12.2-55.1%,并通过抑制活性氧(ROS)的产生,损害中性粒细胞胞外陷阱(NET)的形成。RNA-seq分析显示,ENR破坏肾脏的造血分化,下调gata2(3.5倍),上调凋亡相关基因(bax, 3.3倍)。关键是,ENR通过调节下丘脑-垂体-甲状腺(HPT)轴,使甲状腺素(T3)水平降低29.9%,促甲状腺素释放激素(TRH)水平降低13.5%,间接损害中性粒细胞功能。这些内分泌干扰与ROS生成能力下降(与对照组相比减少49.8%)和对致病性感染的防御能力下降相关,亲水气单胞菌感染后死亡率高28%,SVCV感染后死亡率高17.6%。值得注意的是,体外ENR暴露没有显示直接的中性粒细胞毒性,突出了系统内分泌-免疫串扰的中心地位。这项工作提供了将慢性ENR暴露与HPT轴介导的中性粒细胞抑制联系起来的第一个证据,为水生环境中抗生素持久性的生态风险提供了重要的见解。有限的研究探讨了恩诺沙星对水生环境免疫的影响。本研究表明,恩诺沙星通过损害甲状腺信号和肾脏微环境来破坏斑马鱼的免疫防御,从而对水生生态系统构成潜在威胁。本文章由计算机程序翻译,如有差异,请以英文原文为准。
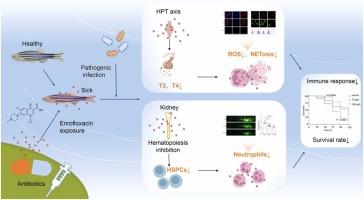
Chronic exposure to environmental concentrations of enrofloxacin impairs neutrophil function in zebrafish via HPT axis disruption and hematopoietic dysregulation
The widespread detection of fluoroquinolone antibiotics like enrofloxacin (ENR) in aquatic ecosystems raises significant concerns about ecological and immunological impacts. While acute ENR toxicity is documented, the immunotoxic effects of chronic, environmentally relevant exposure remain poorly understood. This study investigates the mechanisms by which long-term ENR exposure (28 days at 10–100 μg/L,concentrations reflecting common pollution levels in freshwater systems near aquaculture facilities and extreme contamination scenarios due to wastewater treatment failure) compromises zebrafish immunity, focusing on neutrophil dysfunction. Our results demonstrate that ENR reduces neutrophil counts by 12.2–55.1 % in hematopoietic organs (blood, kidney, spleen) and impairs neutrophil extracellular trap (NET) formation by suppressing reactive oxygen species (ROS) production. RNA-seq analysis reveals ENR disrupts hematopoietic differentiation in the kidney, downregulating gata2 (3.5-fold) and upregulating apoptosis-related genes (bax, 3.3-fold). Crucially, ENR indirectly impairs neutrophil function by dysregulating the hypothalamic-pituitary-thyroid (HPT) axis, reducing thyroxine (T3) levels by 29.9 % and thyrotropin-releasing hormone (TRH) by 13.5 %. These endocrine disruptions correlate with diminished ROS generation capacity (49.8 % reduction vs. controls) and compromised defenses against pathogenic infection, evidenced by 28 % higher mortality after Aeromonas hydrophila infection and 17.6 % higher mortality after SVCV infection. Notably, in vitro ENR exposure showed no direct neutrophil toxicity, highlighting the centrality of systemic endocrine-immune crosstalk. This work provides the first evidence linking chronic ENR exposure to HPT axis-mediated neutrophil suppression, offering critical insights into the ecological risks of antibiotic persistence in aquatic environments.
Synopsis
Limited studies have explored enrofloxacin's impact on immunity in aquatic environments. This study demonstrates that enrofloxacin disrupts zebrafish immune defenses by impairing thyroid signaling and the renal microenvironment, thereby posing a potential threat to aquatic ecosystems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


