吲哚光催化剂和仲胺配体使镍-光氧化还原C(sp2) -杂原子偶联
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
镍-光化学C(sp2) -杂原子偶联反应已成为构建多种分子结构的有力工具。然而,大多数现有的方法依赖于昂贵的光催化剂或专门的配体,限制了它们的实用性和可扩展性。在这里,我们介绍了一种由廉价的吲哚驱动的光催化引发策略,消除了对设计光催化剂的需要。此外,我们证明了高度可调的仲胺配体在促进偶联的同时抑制使Ni催化剂脱离循环的副反应的有效性。我们的方法能够实现广泛的胺化和醚化反应,具有良好的产率和官能团耐受性,为C-N和C-O偶联提供了一个可扩展的平台,该平台依赖于现成的光催化剂和成本效益高的模块化配体。最后,机理研究表明,该反应是通过非常规芳基自由基引发的Ni(I/III)催化循环进行的,与传统的Ni光氧化还原过程不同。这种引发模式中,芳基自由基在温和的条件下与有机金属催化相容,有望成为除ni催化过程之外的其他合成转化的可推广平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
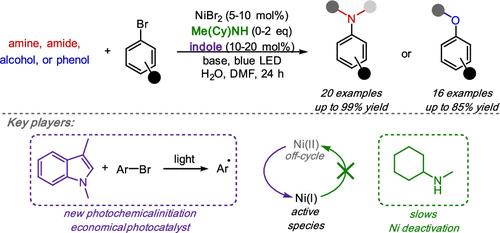
Indole Photocatalysts and Secondary Amine Ligands Enable Nickel-Photoredox C(sp2)–Heteroatom Couplings
Nickel-photochemical C(sp2)–heteroatom coupling reactions have emerged as a powerful tool for constructing diverse molecular architectures. However, most existing methods rely on expensive photocatalysts or specialized ligands, limiting their practicality and scalability. Here, we introduce a photocatalytic initiation strategy driven by inexpensive indoles, eliminating the need for designer photocatalysts. Additionally, we demonstrate the effectiveness of highly tunable secondary amine ligands in facilitating coupling while suppressing side reactions that sequester the Ni catalyst off-cycle. Our approach enables a broad range of amination and etherification reactions with good yields and functional group tolerance, providing a scalable platform for C–N and C–O couplings that relies on a readily available photocatalyst and cost-effective, modular ligands. Finally, mechanistic investigations suggest that the reaction operates via an unconventional aryl radical-initiated Ni(I/III) catalytic cycle, distinguishing it from traditional Ni-photoredox processes. This initiation mode, in which aryl radicals are generated under mild conditions compatible with organometallic catalysis, is expected to serve as a generalizable platform for other synthetic transformations beyond Ni-catalyzed processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


