重金属存在下非生物Fe(II)氧化生成的Fe氧化物对有机物的固存和对磷酸盐的吸附
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
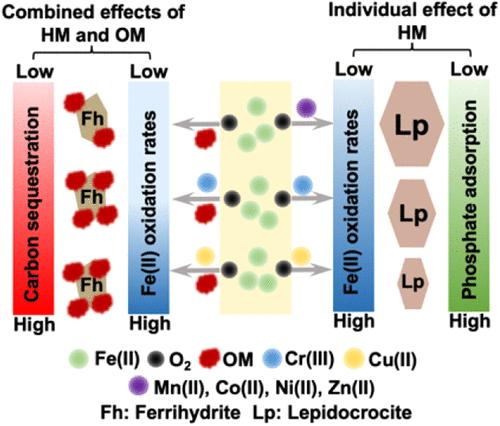
铁(II)氧化沉淀影响土壤/沉积物中有机质(OM)和养分的命运,但通常共存的重金属(hm)的影响仍未得到充分研究。在此,我们研究了中性pH下HM和OM对Fe(II)氧化沉淀的单独和联合影响,以及相关的HM和有机物的固存,以及由此产生的Fe氧化物对磷的吸附。不同的HMs对Fe(II)的氧化有不同的影响,这归因于它们的固有性质,如水解常数、离子电荷和半径。具体来说,Cu(II)或Cr(III)加速了Fe(II)的氧化,并促进了铁氧化物结晶不良的形成,其加速速度取决于它们的浓度,而Mn(II)、Co(II)、Ni(II)或Zn(II)的影响可以忽略不计。同时,HMs的固碳效率遵循Cr(III) >的趋势;锌(II)在铜(II)在有限公司(II)在镍(II)在锰(II)。Cu(II)或Cr(III)通过促进Fe-OM缔合的聚集来增强有机物的固存。在Cu(II), Cr(III)和/或OM存在下生成的铁氧化物由于其小粒径而表现出更高的磷酸盐吸附。我们的研究结果强调了以前被低估的HMs对Fe(II)氧化的重要性及其对碳和磷酸盐保留的潜在影响,这应该在有机物和磷酸盐封存的预测模型中考虑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced Organic Matter Sequestration and Phosphate Adsorption by Fe Oxides Generated from Abiotic Fe(II) Oxidation in the Presence of Heavy Metals
Fe(II) oxidation–precipitation affects the fate of organic matter (OM) and nutrients in soils/sediments, but the effects of commonly coexisting heavy metals (HMs) remain understudied. Herein, we investigated the individual and combined effects of HMs and OM on Fe(II) oxidation–precipitation at neutral pH, along with the associated HM and organic matter sequestration, as well as phosphorus adsorption by the resultant Fe oxides. Various HMs exerted different influences on Fe(II) oxidation, which were attributed to their intrinsic properties, such as hydrolysis constant, ionic charge, and radius. Specifically, Cu(II) or Cr(III) accelerated Fe(II) oxidation and facilitated the formation of poorly crystalline Fe oxides, with the rate of acceleration depending on their concentrations, whereas Mn(II), Co(II), Ni(II), or Zn(II) showed negligible effects. Meanwhile, the sequestration efficiency of HMs followed the trend of Cr(III) > Zn(II) > Cu(II) > Co(II) > Ni(II) > Mn(II). Either Cu(II) or Cr(III) enhanced organic matter sequestration via promoting aggregation of Fe–OM associations. Fe oxides generated in the presence of Cu(II), Cr(III), and/or OM exhibited elevated phosphate adsorption due to their small particle sizes. Our results highlight the previously underappreciated importance of HMs on Fe(II) oxidation and their potential effects on carbon and phosphate retention, which should be considered in predictive models of organic matter and phosphate sequestration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


