基于盐酸介质中离子溶剂化的各种有机溶剂金(III)可萃取性机器学习预测
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从废旧电器电子设备中选择性回收黄金已引起越来越多的关注。金(III)以四氯金酸(HAuCl4)的形式存在于盐酸介质中,可以用各种有机溶剂(如酮类和醚类)提取。然而,预测金(III)可萃取性的溶剂决定性因素尚未得到证实。本研究考察了79种溶剂对Au(III)提取率与溶剂性质的关系。在阈值为80%的5.0 M HCl条件下,根据溶剂Hansen溶解度参数与Au(III)提取率之间的关系,对金(III)的可提取率进行了分类,准确率为93.7%。为了预测Au(III)的可提取性,以RDKit获得的溶剂分子描述符之间的关系为解释变量,以Au(III)的提取率为客观变量,构建了机器学习模型。通过选取与Au(III)提取率相关性较强的描述符,35个描述符的非线性支持向量回归模型检验数据的平方相关系数R2为0.943。再次进行机器学习,并使用实际溶剂的类导体筛选模型从溶剂结构中获得表面电荷密度描述符。包含氢键受体描述符作为附加描述符的28个描述符的非线性支持向量回归模型的检验数据R2值为0.899,略低于35个描述符的模型。使用另外六种溶剂的实验验证证实了模型的准确性,预测值与测量的提取百分比非常匹配。本文章由计算机程序翻译,如有差异,请以英文原文为准。
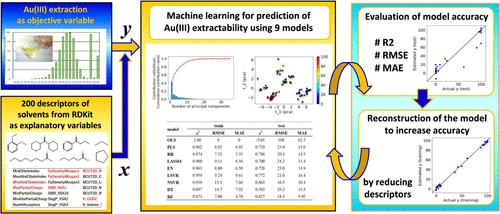
Machine Learning Prediction of Au(III) Extractability of Various Organic Solvents Based on Ion Solvation in Hydrochloric Acid Media
Selective recovery of gold from waste electrical and electronic equipment has attracted increasing attention. Au(III), which is present as tetrachloroauric acid (HAuCl4) in hydrochloric acid media, can be extracted by using various organic solvents, such as ketones and ethers. However, the decisive factors of the solvent for predicting Au(III) extractability have not been confirmed. In the present study, the relationship between the extraction percentage of Au(III) and the properties of the solvent was investigated for 79 types of solvents. Based on the relationships between the Hansen solubility parameters of the solvent and the extraction percentage of Au(III) in 5.0 M HCl with a threshold of 80%, the extractability was classified with 93.7% accuracy. To predict the Au(III) extractability, a machine learning model was constructed using the relationship between the molecular descriptors of the solvents obtained from RDKit as explanatory variables and the extraction percentage of Au(III) as the objective variable. By selection of the descriptors that showed a strong correlation with the Au(III) extraction percentage, the squared correlation coefficient (R2) of the test data for the nonlinear support vector regression model using 35 descriptors was 0.943. Machine learning was performed again with the addition of surface charge density descriptors obtained from the solvent structure using the conductor-like screening model for real solvents. The R2 value of the test data for the nonlinear support vector regression model using 28 descriptors, including the hydrogen bond acceptor descriptor as an additional descriptor, was 0.899, which was slightly lower than that of the model with 35 descriptors. Experimental validation using six additional solvents confirmed the model’s accuracy, with predicted values closely matching the measured extraction percentages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


