1T/2H-MoS2阴极对过氧单硫酸盐的持续活化增强了有机污染物的去除:基于表面mo -过氧单硫酸盐配合物的性能和非自由基活化机制
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
二硫化钼是一种有效的过氧单硫酸盐(PMS)活化催化剂,但其可重复使用性较差,阻碍了其实际应用。为了解决这一问题,我们将多相1T/2H-MoS2固定在碳纤维布(1T/2H-MoS2@CFC)上,并利用其作为阴极,因为阴极提供的电子可以再生Mo催化位点,从而持续激活PMS。此外,电化学1T/2H-MoS2@CFC阴极/PMS系统通过耦合PMS活化和电吸附有机物,增强了对水中有机污染物的去除性能。通过实验和理论的综合研究,我们发现1T/2H-MoS2的负极化使1T/2H-MoS2中Mo活性位点的静电电位更正,吸附的HSO5−的lS-O更长,而正极化则相反。因此,1T/2H-MoS2阴极比阳极获得更高的PMS激活效率,并诱导形成非自由基的表面Mo-PMS配合物,这与1T/2H-MoS2阳极诱导的激活途径不同,该途径涉及•OH, SO4•-和1O2。最后,对1T/2H-MoS2@CFC阴极/PMS系统在不同实际水基质中的降解性能进行了评估,发现在所有情况下都表现良好。本研究结果为水净化提供了一种高效、经济的电化学氧化系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
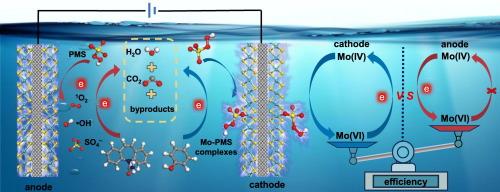
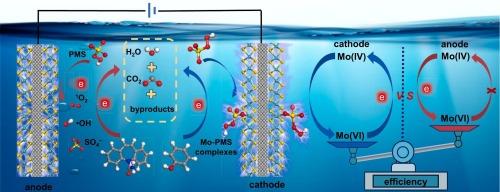
Sustainable peroxymonosulfate activation by 1T/2H-MoS2 cathode for enhanced removal of organic pollutants: Performance and nonradical activation mechanism based on surface Mo-peroxymonosulfate complexes
MoS2 is an effective catalyst for peroxymonosulfate (PMS) activation, but its poor reusability hinders the practical application. To address this issue, we immobilized multiphasic 1T/2H-MoS2 onto a carbon fiber cloth (1T/2H-MoS2@CFC) and utilized it as the cathode to sustainably activate PMS because electrons supplied by cathode can regenerate Mo catalytic sites. In addition, the electrochemical 1T/2H-MoS2@CFC cathode/PMS system manifests enhanced removal performance of aqueous organic pollutants by coupling PMS activation with electrosorption of organics. Through integrated experimental and theoretical investigations, we found that negative polarization of 1T/2H-MoS2 makes the electrostatic potential of Mo active sites in 1T/2H-MoS2 more positive and lS-O of adsorbed HSO5− longer, while positive polarization does the opposite. Accordingly, 1T/2H-MoS2 cathode attains higher PMS activation efficiency than the anode and induces formation of nonradical surface Mo-PMS complexes, differing from the activation pathway induced by 1T/2H-MoS2 anode which involves •OH, SO4•- and 1O2. Finally, the degradation performance of 1T/2H-MoS2@CFC cathode/PMS system in different real water matrices was assessed and it was found to perform effectively in all cases. Our findings offer an efficient and economical electrochemical oxidative system for water decontamination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


