Talal Beidas, Luther Light, Caroline Carrico, Shayne Kondor, Prasanth Ravi, Yotom A Rabinowitz
下载PDF
{"title":"印刷外包正颌外科夹板内部:一个尺寸验证过程的点护理印刷。","authors":"Talal Beidas, Luther Light, Caroline Carrico, Shayne Kondor, Prasanth Ravi, Yotom A Rabinowitz","doi":"10.1186/s41205-025-00276-9","DOIUrl":null,"url":null,"abstract":"<p><strong>Background: </strong>Both outsourcing virtual surgery planning and 3D printed splint fabrication have become the standard in the field of orthognathic surgery. In-house (IH) adaptation of these presurgical operations requires compliance with regulatory bodies when designing and manufacturing medical-grade products. The purpose of this study is to evaluate the dimensional accuracy of IH 3D printed orthognathic surgical splints within a hybrid workflow for externally designed splints.</p><p><strong>Materials and methods: </strong>An in vitro study was conducted utilizing an outsourced (OS) orthognathic surgical splint file from a previously treated patient. The control group included the splint's original standard tessellation language (STL) file. Experimental groups included: splint (a) milled with zirconia, (b) 3D printed on a Phrozen 4 K (commercial printer), (c) 3D printed on Formlabs 3B+ (Formlabs 3B + have track record of having FDA cleared materials and workflows in dental applications and have potential materials that could be used for IH manufacturing for orthognathic splints upon further testing and FDA clearance). Surface area analysis was performed using 3-Matic (Materialise© Mimics Software) to generate root mean square (RMS) values between digital copies of the splints and their corresponding original STL files.</p><p><strong>Results: </strong>The RMS error in equipment processing and analysis was measured at 0.10 mm. Accounting for this error, the 3D-printed splints exhibited an average RMS of 0.20 mm for both the Formlabs 3B + and the Phrozen 4 K. No statistically significant difference was found between splints from different printers or replicas.</p><p><strong>Conclusion: </strong>This study presents a verification process for providers to verify the geometric stability and reproducibility of their IH-printed orthognathic splints (RMS 0.20 mm). Clinicians may find this study useful when crafting a regulatory-compliant process for the IH manufacturing of OS orthognathic surgery splints.</p>","PeriodicalId":72036,"journal":{"name":"3D printing in medicine","volume":"11 1","pages":"24"},"PeriodicalIF":3.1000,"publicationDate":"2025-06-06","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12143043/pdf/","citationCount":"0","resultStr":"{\"title\":\"Printing outsourced orthognathic surgical splints in-house: a dimensional verification process for point-of-care printing.\",\"authors\":\"Talal Beidas, Luther Light, Caroline Carrico, Shayne Kondor, Prasanth Ravi, Yotom A Rabinowitz\",\"doi\":\"10.1186/s41205-025-00276-9\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p><strong>Background: </strong>Both outsourcing virtual surgery planning and 3D printed splint fabrication have become the standard in the field of orthognathic surgery. In-house (IH) adaptation of these presurgical operations requires compliance with regulatory bodies when designing and manufacturing medical-grade products. The purpose of this study is to evaluate the dimensional accuracy of IH 3D printed orthognathic surgical splints within a hybrid workflow for externally designed splints.</p><p><strong>Materials and methods: </strong>An in vitro study was conducted utilizing an outsourced (OS) orthognathic surgical splint file from a previously treated patient. The control group included the splint's original standard tessellation language (STL) file. Experimental groups included: splint (a) milled with zirconia, (b) 3D printed on a Phrozen 4 K (commercial printer), (c) 3D printed on Formlabs 3B+ (Formlabs 3B + have track record of having FDA cleared materials and workflows in dental applications and have potential materials that could be used for IH manufacturing for orthognathic splints upon further testing and FDA clearance). Surface area analysis was performed using 3-Matic (Materialise© Mimics Software) to generate root mean square (RMS) values between digital copies of the splints and their corresponding original STL files.</p><p><strong>Results: </strong>The RMS error in equipment processing and analysis was measured at 0.10 mm. Accounting for this error, the 3D-printed splints exhibited an average RMS of 0.20 mm for both the Formlabs 3B + and the Phrozen 4 K. No statistically significant difference was found between splints from different printers or replicas.</p><p><strong>Conclusion: </strong>This study presents a verification process for providers to verify the geometric stability and reproducibility of their IH-printed orthognathic splints (RMS 0.20 mm). Clinicians may find this study useful when crafting a regulatory-compliant process for the IH manufacturing of OS orthognathic surgery splints.</p>\",\"PeriodicalId\":72036,\"journal\":{\"name\":\"3D printing in medicine\",\"volume\":\"11 1\",\"pages\":\"24\"},\"PeriodicalIF\":3.1000,\"publicationDate\":\"2025-06-06\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12143043/pdf/\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"3D printing in medicine\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://doi.org/10.1186/s41205-025-00276-9\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"3D printing in medicine","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.1186/s41205-025-00276-9","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Printing outsourced orthognathic surgical splints in-house: a dimensional verification process for point-of-care printing.
Background: Both outsourcing virtual surgery planning and 3D printed splint fabrication have become the standard in the field of orthognathic surgery. In-house (IH) adaptation of these presurgical operations requires compliance with regulatory bodies when designing and manufacturing medical-grade products. The purpose of this study is to evaluate the dimensional accuracy of IH 3D printed orthognathic surgical splints within a hybrid workflow for externally designed splints.
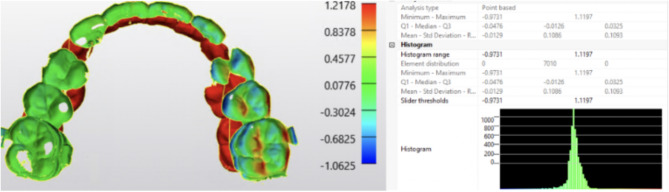
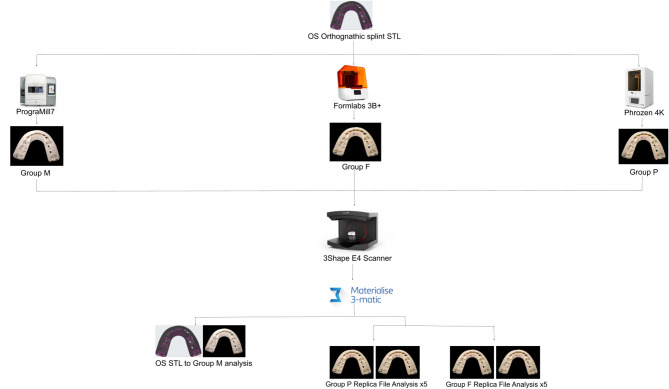
Materials and methods: An in vitro study was conducted utilizing an outsourced (OS) orthognathic surgical splint file from a previously treated patient. The control group included the splint's original standard tessellation language (STL) file. Experimental groups included: splint (a) milled with zirconia, (b) 3D printed on a Phrozen 4 K (commercial printer), (c) 3D printed on Formlabs 3B+ (Formlabs 3B + have track record of having FDA cleared materials and workflows in dental applications and have potential materials that could be used for IH manufacturing for orthognathic splints upon further testing and FDA clearance). Surface area analysis was performed using 3-Matic (Materialise© Mimics Software) to generate root mean square (RMS) values between digital copies of the splints and their corresponding original STL files.
Results: The RMS error in equipment processing and analysis was measured at 0.10 mm. Accounting for this error, the 3D-printed splints exhibited an average RMS of 0.20 mm for both the Formlabs 3B + and the Phrozen 4 K. No statistically significant difference was found between splints from different printers or replicas.
Conclusion: This study presents a verification process for providers to verify the geometric stability and reproducibility of their IH-printed orthognathic splints (RMS 0.20 mm). Clinicians may find this study useful when crafting a regulatory-compliant process for the IH manufacturing of OS orthognathic surgery splints.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


