携带免疫检查点配体的胰岛素前特异性调节性T细胞衍生外泌体可以抑制1型糖尿病的自身免疫反应
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
背景1型糖尿病(T1D)涉及胰腺β (β)细胞的选择性破坏,主要是通过浸润CD8+ T细胞。这些CD8+ T细胞也表达免疫检查点分子(ICMs),可被免疫检查点配体(ICLs)或β细胞特异性Tregs靶向。在这里,我们结合这两种方法来抑制T1D的自身免疫反应。方法采用流式细胞术对40例新近发病的T1D患者和20例年龄匹配的健康人外周血CD8+ T细胞上的各种ICMs进行了分析。从同一受试者中分离treg,并在体外用胰岛素前原(PPI)刺激。从ppi特异性Tregs中分离出外泌体,并通过western blotting、透射电镜、zeta电位和粒度分析对其进行了表征。根据ICM谱图,使用外周CD8+ T细胞上表达的3种最丰富的ICM (PD-1、TIGIT、BTLA)对应的icl来装载外泌体。通过各种体外和体内方法进一步评估装载icl的外泌体(PPI-T-EXOL)的疗效。结果PPI-T-EXOL能抑制自体CD8+和CD4+ Teff细胞的增殖。注入PPI-T-EXOL的PPI-T-EXOL和PPI-Tregs显著下调了自体ppi脉冲CD8+ T细胞的活化和细胞毒电位。这些Tregs还减少了CD8+ T细胞介导的人1.1B4 β细胞系的凋亡。在stz诱导的糖尿病C57BL/6小鼠中,小鼠特异性icl负载外泌体延迟了高血糖的发生,特别是在糖尿病发病前给药时,并通过抑制血管周围淋巴细胞胰岛内浸润延长了它们的生存期。结论sicl负载的ppi - treg衍生外泌体可以抑制β细胞特异性T细胞反应,为T1D的治疗提供了有希望的干预措施。本文章由计算机程序翻译,如有差异,请以英文原文为准。
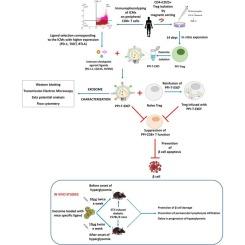
Preproinsulin-specific regulatory T cell-derived exosomes loaded with immune-checkpoint ligands can suppress autoimmune responses in type 1 diabetes
Background
Type 1 diabetes (T1D) involves selective destruction of pancreatic beta (β) cells, mainly by infiltrating CD8+ T cells. These CD8+ T cells also express immune-checkpoint molecules (ICMs) that can be targeted by immune-checkpoint ligands (ICLs) or β cell-specific Tregs. Here, we combined both approaches to suppress autoimmune responses in T1D.
Methods
We first performed profiling of various ICMs on peripheral CD8+ T cells in 40 recent-onset T1D and 20 age-matched healthy subjects by flow cytometry. Tregs were isolated from the same subjects and stimulated with preproinsulin (PPI) in vitro. Exosomes were isolated from PPI-specific Tregs and characterized by western blotting, transmission electron microscopy, zeta potential, and particle size analysis. Based on ICM profile, ICLs corresponding to the 3 most abundant ICMs (PD-1, TIGIT, BTLA) expressed on the peripheral CD8+ T cells were used for loading exosomes. The efficacy of ICL-loaded exosomes (PPI-T-EXOL) was further assessed by various in vitro and in vivo approaches.
Results
The PPI-T-EXOL inhibited the proliferation of autologous CD8+ and CD4+ Teff cells. The PPI-T-EXOL and PPI-Tregs infused with PPI-T-EXOL significantly downregulated the activation and cytotoxic potential of autologous PPI-pulsed CD8+ T cells. These Tregs also reduced CD8+ T cell-mediated apoptosis of human 1.1B4 β-cell line. In STZ-induced diabetic C57BL/6 mice, the mice-specific ICL-loaded exosomes delayed the onset of hyperglycemia, particularly when administered before the onset of diabetes and prolonged their survival by inhibiting perivascular lymphocytic intra-islet infiltration.
Conclusions
ICL-loaded PPI-Treg-derived exosomes can suppress β cell-specific T cell responses, offering a promising therapeutic intervention in T1D.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


