人肝嵌合小鼠匹罗卡品氧化和水解代谢的研究
IF 2.2
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
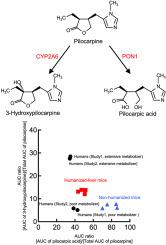
人源化肝脏嵌合小鼠(人源化肝脏小鼠)是预测人体内药代动力学的一种有吸引力的替代方法。我们的目的是研究CYP2A6和PON1在人源肝小鼠匹罗卡品生物转化中的作用。单次口服剂量为10 mg/kg后,在人源化肝脏小鼠中观察到比非人源化小鼠更高的血浆浓度匹罗卡品和3-羟基匹罗卡品。与在非人源化小鼠中观察到的3-羟基匹罗卡品和匹罗卡酸相比,在人源化肝脏小鼠中,匹罗卡品的曲线下面积(AUC)与总AUC的比值更接近于在CYP2A6代谢表型广泛的人类中观察到的结果。人源化小鼠肝微粒体中的匹罗卡品3-羟化酶活性高于非人源化小鼠,而血浆中的匹罗卡品水解酶活性在两组之间具有可比性。人源化肝小鼠肝微粒体中CYP2A6水平与匹罗卡品3-羟化酶活性呈正相关(P = 0.04)。此外,在人CYP2A6或PON1抑制剂存在的情况下,人源化肝脏小鼠的肝微粒体3-羟化酶和血浆匹罗卡品水解酶活性均下降。在人源化肝小鼠中,匹罗卡品的血浆代谢物谱与人类相似,这突出了人源化肝小鼠用于研究人类cyp2a6和pon1依赖性药物生物转化的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigation of pilocarpine oxidative and hydrolytic metabolism in chimeric mice with humanized liver
Chimeric mice with humanized liver (humanized-liver mice) are an attractive alternative to conventional animals for predicting pharmacokinetics in humans. We aimed to investigate the role of CYP2A6 and PON1 on pilocarpine biotransformation in humanized-liver mice. Following a single oral dose of 10 mg/kg, higher plasma concentrations of pilocarpine and 3-hydroxypilocarpine were observed in humanized-liver mice than in non-humanized mice. The ratios of area under the curve (AUC) to total AUC for pilocarpine in humanized-liver mice were more similar to those observed in humans with extensive metabolizer phenotypes for CYP2A6 compared to those in non-humanized mice for both 3-hydroxypilocarpine and pilocarpic acid. Pilocarpine 3-hydroxylase activity in liver microsomes was higher in humanized-liver mice than in non-humanized mice, whereas the pilocarpine hydrolase activity in plasma was comparable between both groups. CYP2A6 levels and pilocarpine 3-hydroxylase activities in liver microsomes from humanized-liver mice were correlated (P = 0.04). Furthermore, both hepatic microsomal 3-hydroxylase and plasma hydrolase activities of pilocarpine in humanized-liver mice decreased in the presence of respective human CYP2A6 or PON1 inhibitors. The plasma metabolite profiles of pilocarpine in humanized-liver mice were similar to those in humans, highlighting the suitability of humanized-liver mice for investigating CYP2A6-and PON1-dependent drug biotransformation in humans.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.80
自引率
9.50%
发文量
50
审稿时长
69 days
期刊介绍:
DMPK publishes original and innovative scientific papers that address topics broadly related to xenobiotics. The term xenobiotic includes medicinal as well as environmental and agricultural chemicals and macromolecules. The journal is organized into sections as follows:
- Drug metabolism / Biotransformation
- Pharmacokinetics and pharmacodynamics
- Toxicokinetics and toxicodynamics
- Drug-drug interaction / Drug-food interaction
- Mechanism of drug absorption and disposition (including transporter)
- Drug delivery system
- Clinical pharmacy and pharmacology
- Analytical method
- Factors affecting drug metabolism and transport
- Expression of genes for drug-metabolizing enzymes and transporters
- Pharmacogenetics and pharmacogenomics
- Pharmacoepidemiology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


