尼罗河红基共价有机骨架作为C-H键功能化的光催化剂
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
寻找基于共价有机框架(COFs)的高效光催化剂是一个越来越受关注的领域。然而,这些非均相光催化剂的发展受到用于构建这些材料的连接剂的对称性限制。在此,我们报告了一种亚胺基2D-COF的直接合成,NR0.17-COF,它通过后修饰与NR-炔支架结合了尼罗河红(NR)单元。该框架在各种光氧化还原催化的C-H功能化反应中表现出显著的光催化活性,证明了在温和条件和低能光下直接功能化有机分子中常见键的能力。NR0.17-COF显示出显著的多功能性,可以有效地从不同的自由基前体生成芳基、硫和氮自由基,同时保持良好的官能团耐受性。此外,我们的多相光催化剂通过解决可扩展性和可回收性等关键挑战,超越了传统的均相系统,使反应规模增加了10倍,并实现了多达6次的回收和再利用。这一进展极大地增强了COF合成后修饰在有机合成中的实际应用潜力,标志着光催化技术向前迈出了一大步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
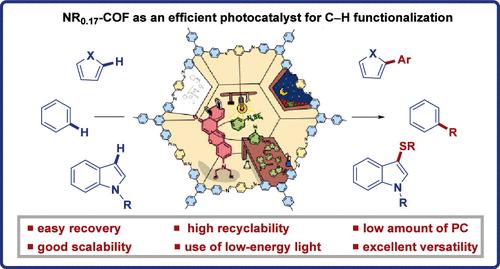
Nile Red-Based Covalent Organic Framework as a Photocatalyst for C–H Bond Functionalization
The search for efficient photocatalysts based on covalent organic frameworks (COFs) is an area of increasing interest. However, the development of these heterogeneous photocatalysts is hindered by the symmetry restrictions of the linkers used to construct these materials. Herein, we report the straightforward synthesis of an imine-based 2D-COF, NR0.17-COF, which incorporates a Nile Red (NR) unit via postmodification with a NR-alkyne scaffold. This framework exhibits remarkable photocatalytic activity across various photoredox-catalyzed C–H functionalization reactions, demonstrating the ability to directly functionalize prevalent bonds in organic molecules under mild conditions and with low-energy light. The NR0.17-COF showcases notable versatility, effectively generating aryl, sulfur, and nitrogen radicals from different radical precursors while maintaining good functional group tolerance. Moreover, our heterogeneous photocatalyst outperforms traditional homogeneous systems by addressing critical challenges such as scalability and recyclability, allowing for a 10-fold increase in the reaction scale and enabling recovery and reuse up to six times. This advancement significantly enhances the potential of COF postsynthetic modification for practical applications in organic synthesis, which marks a substantial step forward in photocatalytic technology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


