基于聚磷酸和抗氧化肽的凝聚体递送miRNA
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
基于RNA的治疗往往受到低RNA稳定性和细胞质递送效率的阻碍。通过液-液相分离(LLPS)形成的凝聚液滴由于其凝聚性和流动性,在载药和将生物大分子输送到细胞质中的转染效率方面具有很大的潜力。在这里,我们开发了一种聚簇状液滴作为递送载体,由六偏磷酸钠(SHMP)和抗氧化肽SS-31的LLPS形成,然后装载microRNA-223 (miRNA-223)作为聚簇状人工细胞(Coac@miR)。此外,在Coac@miR (EMCoac@miR)上的红细胞膜涂层被用来保护miRNA-223在血液转移过程中不被核糖核酸酶降解。聚簇状人工细胞显示miRNA-223的细胞质传递效率比单独的miRNA-223高10倍。在急性肺损伤(ALI)小鼠模型中,我们发现气管内注射(i.t.) Coac@miR和静脉注射(i.v.) EMCoac@miR都可以通过将巨噬细胞重编程为抗炎(M2)表型、抑制炎症因子和缓解ROS应激来缓解ALI。这项工作为polyP-peptide-based cocervate人工细胞内的mirna提供了一种新的递送系统,展示了免疫相关疾病和炎症性疾病的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
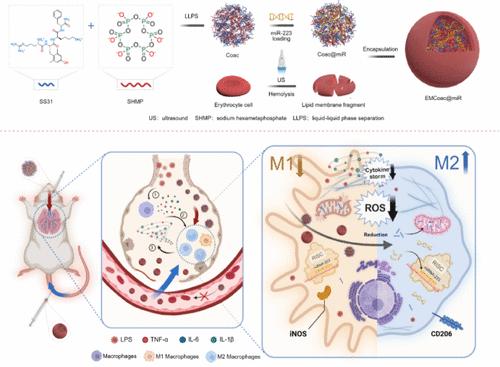
Polyphosphate- and Antioxidant Peptide-Based Coacervate Delivers miRNA
RNA-based therapies are often hampered by low RNA stability and cytoplasmic delivery efficiency. Coacervate droplets formed by liquid–liquid phase separation (LLPS) exhibit great potential in drug loading and transfection efficiency for delivering biomacromolecules into the cytoplasm due to their condensed and fluid nature. Here, we developed a type of coacervate droplet as the delivery vector formed by the LLPS of sodium hexametaphosphate (SHMP) and antioxidant peptide SS-31, followed by loading with microRNA-223 (miRNA-223) as a coacervate artificial cell (Coac@miR). In addition, an erythrocyte membrane coating on the Coac@miR (EMCoac@miR) is employed to shield the miRNA-223 from ribonuclease degradation during blood transfer. The coacervate artificial cells demonstrate increased cytoplasmic delivery efficiency of miRNA-223 by 10-fold higher than the miRNA-223 alone. With acute lung injury (ALI) mouse model, we find that both intratracheal injection (i.t.) of Coac@miR and intravenous injection (i.v.) of EMCoac@miR could alleviate ALI by reprogramming macrophages to an anti-inflammatory (M2) phenotype, inhibiting inflammatory factors, and relieving ROS stress. This work provides a novel delivery system for miRNAs within polyP-peptide-based coacervate artificial cells, demonstrating therapeutic potential for immune-related and inflammatory diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


