生物衍生的4,4 ' -二甲基联苯可扩展合成4,4 ' -二羧酸二甲基联苯
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在50 mL的钛反应器中,采用Mid-Century (MC)工艺条件,成功地证明了4,4 ' -二甲基联苯(DMBP)(来源于生物衍生的2-甲基呋喃的对二甲苯类似物)均相催化氧化为4,4 ' -联苯二羧酸(BPDA)。为了适应MC工艺,固体DMBP在140°C下外部熔化,并在氧气的作用下连续进入反应器。该工艺可制得数十克BPDA,纯度为98.7%。相反,由于安全操作所需的亚化学计量O2/DMBP比,将所有固体DMBP最初送入反应器的间歇氧化产生纯度为~ 80%的BPDA。DMBP和O2的共同投注维持了化学计量O2/DMBP的比率或更高,避免了O2饥饿而不超过安全气相O2浓度。BPDA很容易用甲醇硫酸酯化制得聚合物级二甲基联苯4,4 ' -二羧酸酯(BPDC, 99%)。BPDC的熔点比BPDA低,因此适合在较温和的温度下进行熔融聚合。DMBP氧化生产可再生BPDA的MC过程化学成功演示,广泛应用于工业生产对苯二甲酸(BPDA类似物),降低了DMBP氧化规模化的经济风险。由于化石原料制备占MC过程中从摇篮到大门的全球变暖潜势的大部分(~ 75%),通过类似的途径使用生物衍生原料制造BPDC有可能显著减轻碳足迹。本文章由计算机程序翻译,如有差异,请以英文原文为准。
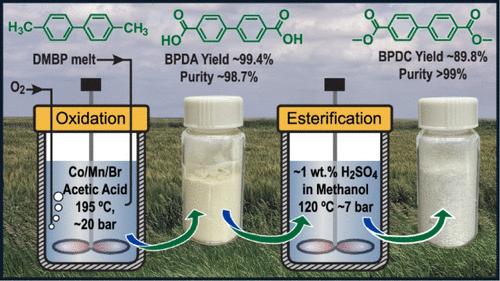
Scalable Synthesis of Dimethyl Biphenyl-4,4′-Dicarboxylate from Bio-Derived 4,4′-Dimethylbiphenyl
The homogeneous catalytic oxidation of 4,4′-dimethylbiphenyl (DMBP), an analogue of p-xylene sourced from bioderived 2-methylfuran, to 4,4′-biphenyldicarboxylic acid (BPDA) has been successfully demonstrated in a 50 mL titanium reactor using Mid-Century (MC) process conditions. To adapt to the MC process, the solid DMBP was melted externally at 140 °C and cofed continuously into the reactor with oxygen. Such an operation can produce tens of grams of BPDA with 98.7% purity. In contrast, batch oxidation in which all the solid DMBP is fed initially into the reactor produces BPDA with ∼80% purity due to the substoichiometric O2/DMBP ratios required for safe operation. Cofeeding of DMBP and O2 maintains the stoichiometric O2/DMBP ratio or greater, avoiding O2 starvation without exceeding safe vapor phase O2 concentrations. BPDA was easily esterified using methanolic H2SO4 to make polymer-grade dimethyl biphenyl 4,4′-dicarboxylate (BPDC, >99%). BPDC has a lower melting point than BPDA and hence is amenable to melt polymerization at milder temperatures. The successful demonstration of DMBP oxidation to produce renewable BPDA via MC process chemistry, widely used in industry to produce terephthalic acid (a BPDA analogue), reduces the economic risk of scaling up DMBP oxidation. As fossil-based feedstock preparation accounts for a majority (∼75%) of the cradle-to-gate global warming potential in the MC process, the use of bioderived feedstock for BPDC manufacture via a similar route has the potential to significantly mitigate the carbon footprint.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


