用电化学和计算方法研究含有离子液体电解质的超级电容器的高性能
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
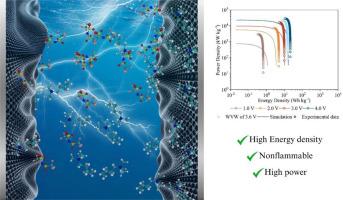
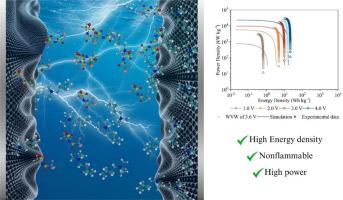
本研究表征了对称硬币电池的电化学行为,其基于交流的电极填充了基于氨的离子液体,由于其大电压窗和环境温度操作,这是一种很有前途的超级电容器电解质。我们采用循环伏安法、单步计时安培法、电化学阻抗谱法和恒流充放电技术对超级电容器的电化学性能进行了全面评价。我们的研究结果强调了高达3.6 V的准矩形电流-电压分布,超过此范围,由于电解质降解引起的法拉第电流变得重要。这些结果揭示了一个次要的法拉第分量,阻抗分析显示了典型的结构良好的双电层(edl)的行为,受离子液体独特性质的影响。我们观察到离子动力学和电容行为与理论模型非常吻合,特别是古德温-科尔尼舍夫平均场理论。该研究强调了基于离子液体的超级电容器实现高性能的潜力,其比电容值达到~ 2000 gf -1,能量和功率输出可与锂离子电池媲美。分子动力学模拟进一步阐明了edl内部的电荷机制、离子迁移和结构相互作用。这些全面的见解验证了丁基三甲基铵双(三氟甲基磺酰基)亚胺[N1114][NTf2]在高级超级电容器应用中的适用性,突出了其在优化条件下提供卓越电化学性能的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
High performance of supercapacitors containing an ionic liquid electrolyte by means of electrochemical and computational studies
This study characterizes the electrochemical behavior of symmetric coin cells with AC-based electrodes filled with the ammonium-based ionic liquid, a promising supercapacitor electrolyte due to its large voltage window and ambient temperature operation. We employed cyclic voltammetry, single-step chronoamperometry, electrochemical impedance spectroscopy, and galvanostatic charge-discharge techniques to evaluate the electrochemical performance of the supercapacitors thoroughly. Our findings highlight the quasi-rectangular current-voltage profile up to 3.6 V, beyond which a faradaic current due to electrolyte degradation becomes significant. These results revealed a minor faradaic component, with impedance analysis showing typical behavior of well-formed electrical double layers (EDLs), influenced by the unique properties of ionic liquids. We observed that the ion dynamics and capacitance behavior align well with theoretical models, particularly the Goodwin-Kornishev mean-field theory. The study underscores the potential of ionic liquid-based supercapacitors to achieve high performance, with specific capacitance values reaching ∼2000 F g-1 and great energy and power output, comparable to lithium-ion batteries. Molecular dynamics simulations further elucidated the charging mechanisms, ion migration, and structural interactions within the EDLs. These comprehensive insights validate the suitability of butyltrimethylammonium bis(trifluoromethylsulfonyl)imide [N1114][NTf2] for advanced supercapacitor applications, highlighting its ability to deliver exceptional electrochemical performance under optimized conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


