超临界CO2中正己烷在金属-有机骨架上的吸附平衡:用势理论测量和热力学建模
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
超临界CO2的吸附过程对于从混合物中分离挥发性有机化合物(VOCs)非常有效,极大地帮助了溶剂的回收和所需物质的纯化。本研究探讨了正己烷在金属有机骨架(MOF-177和ZIF-8)上在超临界二氧化碳(CO2)中的吸附平衡。通过固定床法在不同温度(313-353 K)和压力(10.0-20.0 MPa)下进行吸附测量。利用Dubinin-Astakhov (DA)方程分析吸附行为,该方程为超临界条件下的吸附机理提供了热力学见解。结果表明,吸附量随压力升高而减小,随温度升高而增大,主要受CO2密度和正己烷逸度变化的影响。此外,MOF-177表现出比ZIF-8更大的吸附能力,这与它增加的孔隙体积和表面积有关。DA模型有效地解释了数据,导出的参数(特征吸附能(EVOC)和最大吸附容量(W0,VOC))量化了CO2密度和吸附剂特性的影响。该研究为设计和优化超临界CO2环境中基于吸附的分离工艺提供了重要的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
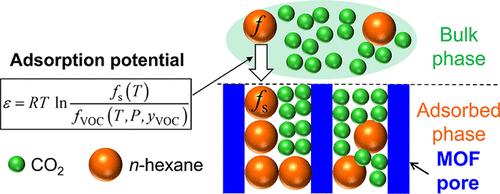
Adsorption Equilibria of n-Hexane on Metal–Organic Frameworks in Supercritical CO2: Measurement and Thermodynamic Modeling with a Potential Theory
The adsorption process using supercritical CO2 is highly effective for separating volatile organic compounds (VOCs) from mixtures, significantly aiding solvent recovery and purification of desired substances. This study explores the adsorption equilibria of n-hexane on metal–organic frameworks (MOFs), namely MOF-177 and ZIF-8, in supercritical carbon dioxide (CO2). Adsorption measurements were obtained through a fixed-bed method across various temperatures (313–353 K) and pressures (10.0–20.0 MPa). The analysis of adsorption behavior utilized the Dubinin–Astakhov (DA) equation, which provides thermodynamic insights into the adsorption mechanisms under supercritical conditions. The results indicate that adsorption capacity decreases with increasing pressure and increases with higher temperature, mainly due to changes in CO2 density and n-hexane fugacity. Additionally, MOF-177 demonstrates greater adsorption capacity than ZIF-8, a difference linked to its increased pore volume and surface area. The DA model effectively explained the data, and the derived parameters (characteristic adsorption energy (EVOC) and maximum adsorption capacity (W0,VOC)) quantified the influences of CO2 density and adsorbent characteristics. This research offers significant insights for designing and optimizing adsorption-based separation processes in supercritical CO2 environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


