酸性肿瘤微环境调节纳米颗粒增强胃癌光免疫治疗
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
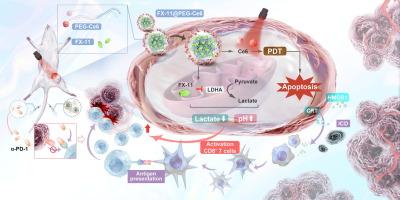
免疫疗法,尤其是抗pd -1抗体(α-PD-1),已经彻底改变了癌症治疗的前景。然而,α-PD-1对胃癌(GC)的应答率仍然相对较低。酸性免疫抑制肿瘤微环境(Tumor Microenvironment, TME)极大地阻碍了α-PD-1的疗效。因此,迫切需要针对GC中酸性TME的治疗策略。目的探讨FX-11@PEG-Ce6对光免疫治疗胃癌疗效的影响。方法制备聚乙二醇- ce6纳米颗粒包裹的FX-11 (FX-11@PEG-Ce6)用于气相色谱光免疫治疗。透射电镜观察其形貌。采用流式细胞术检测树突状细胞成熟水平及CD8+ T细胞中TNF-α、IFN-γ、颗粒酶B水平,评价FX-11@PEG-Ce6光免疫治疗联合α-PD-1体外和体内协同抗肿瘤作用。采用苏木精、伊红染色及主要脏器生化分析,研究FX-11@PEG-Ce6的生物安全性。结果FX-11@PEG-Ce6作为一种纳米平台,具有良好的细胞摄取和肿瘤靶向能力。FX-11@PEG-Ce6引起了显著的免疫原性细胞死亡反应。同时,流式细胞术结果显示FX-11@PEG-Ce6促进了树突状细胞的成熟,增加了t细胞因子的分泌。通过对细胞培养基pH值的检测,发现FX-11@PEG-Ce6可以减轻TME的酸性,从而恢复T细胞的功能,增强CD8+ T细胞的抗肿瘤活性。采用MFC荷瘤小鼠模型。体内实验结果表明FX-11@PEG-Ce6可以缓解酸性TME,帮助根除肿瘤细胞。FX-11@PEG-Ce6显著增强α-PD-1的药效,并表现出优越的生物相容性。结论FX-11@PEG-Ce6-based光动力疗法与免疫疗法联合治疗胃癌具有协同抗肿瘤作用,且具有良好的生物安全性,具有很强的光免疫增强治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acidic tumor microenvironment–modulated nanoparticle potentiates gastric cancer photoimmunotherapy
Introduction
Immunotherapy, especially anti-PD-1 antibodies (α-PD-1), has revolutionized the landscape of cancer treatment. However, the response rate of α-PD-1 for Gastric Cancer (GC) remains relatively low. The acidic immunosuppressive Tumor Microenvironment (TME) greatly hinders the efficacy of α-PD-1. Thus, therapeutic strategies targeting the acidic TME in GC are highly desired.Objectives
This study aimed to investigate the effect of FX-11@PEG-Ce6 on the therapeutic efficacy of photoimmunotherapy for gastric cancer.Methods
We developed FX-11 encapsulated with PEG-Ce6 nanoparticles (FX-11@PEG-Ce6) for GC photoimmunotherapy. The morphology was observed by transmission electron microscopy. Flow cytometry was performed to detect the maturation level of dendritic cells and the levels of TNF-α, IFN-γ, and granzyme B in CD8+ T cells, and to evaluate the synergistic anti-tumor effects of photoimmunotherapy generated by FX-11@PEG-Ce6 in combination with α-PD-1 in vitro and in vivo. The biological safety of FX-11@PEG-Ce6 was studied by haematoxylin and eosin staining and biochemical analysis of major organs.Results
As a type of nanoplatform, FX-11@PEG-Ce6 demonstrated satisfactory cellular uptake and tumor targeting ability. FX-11@PEG-Ce6 provoked significant immunogenic cell death response. Meanwhile, the results of flow cytometry showed that FX-11@PEG-Ce6 facilitated the maturation of dendritic cells and augmented the secretion of T-cell cytokines. Through the detection of the pH of the cell culture medium, it was revealed that FX-11@PEG-Ce6 could alleviate the acidity of the TME, thereby restoring the function of T cells and enhancing the anti-tumor activity of CD8+ T cells. MFC tumor-bearing mouse models were adopted. In vivo results showed that FX-11@PEG-Ce6 could alleviate the acidic TME and help eradicate tumor cells. FX-11@PEG-Ce6 substantially enhance the efficacy of α-PD-1 and exhibit superior biocompatibility.Conclusion
Our results revealed that the combination of FX-11@PEG-Ce6-based photodynamic therapy and immunotherapy could achieve a synergistic antitumor effect with excellent biosafety, presenting great therapeutic potential for enhanced photoimmunotherapy for GC.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


