通过a位调制稳定钙钛矿中的Cu2+,实现CO2对CH4的高效电催化
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
铜基催化剂中的Cu2+可以促进电化学二氧化碳还原反应(ECRR)产生CH4途径的加氢。然而,铜氧化物中的Cu2+是不稳定的,在外加阴极电位的作用下会还原为Cu0。在这项工作中,我们报道了一种a位调制策略来稳定钙钛矿中的Cu2+,从而实现对CH4的高效ECRR。在La2CuO4中引入Ca2+后,得到的LaCa0.4CuO3-δ在ECRR期间是稳定的。我们在-1.30 V下获得了59.6 %±3.8 %的CH4法拉第效率,而在h电池中可逆氢电极中获得了155.0 mA/cm2的分电流密度。DFT计算和原位拉曼光谱结果表明,Cu2+有利于*CH2O加氢生成* ch30,进一步生成CH4。本研究介绍了一种稳定Cu2+的有效策略,并提供了Cu2+在促进ECRR转化为CH4中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
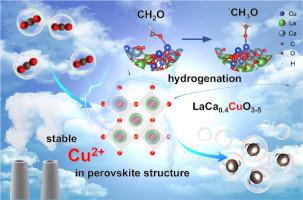
Stabilizing Cu2+ in perovskite via A-site modulation for efficient CO2 electrocatalysis to CH4
Cu2+ in copper-based catalysts can facilitate the hydrogenation of the CH4 production pathway via the electrochemical carbon dioxide reduction reaction (ECRR). However, Cu2+ species in copper oxides are unstable and have been revealed to reduce to Cu0 under the applied cathodic potential. In this work, we reported an A-site modulation strategy to stabilize Cu2+ in perovskite for efficient ECRR to CH4. After the introduction of Ca2+ in La2CuO4, the obtained LaCa0.4CuO3-δ is stable during ECRR. We achieved a 59.6 % ± 3.8 % CH4 faradaic efficiency at -1.30 V versus reversible hydrogen electrode in H-cell and a partial current density of 155.0 mA/cm2 in membrane electrode assembly. DFT calculations and in situ Raman spectroscopy show that Cu2+ facilitates the hydrogenation of *CH2O to *CH3O and the further production of CH4. This work introduces an efficient strategy to stabilize Cu2+ and provides an understanding of Cu2+ in promoting ECRR to CH4.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


