释放拉莫三嗪在纳米管中的潜力:不同溶剂中的DFT, MD模拟,传感特性和药物增强剂
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用密度泛函理论,研究了拉莫三嗪(6-(2,3-二氯苯基)1,2,4-三嗪-3,5-二胺)(DTD)在CC、AlN和BN纳米管上的吸附性能。选择不同的配置进行优化。本研究通过评价纳米管(CC、BN、AlN)在拉莫三嗪(DTD)递送中的作用,解决了对高效药物载体的需求。主要发现包括:PP2 (nh2端)对AlN的吸附能最高(-190.78 kJ/mol);SERS效应证实了dtd与纳米管的结合,MD在水/甲醇中表现出稳定性。在所有情况下,纳米管末端的DTD提供最大的吸附能。对于所有配合物,吸附能变化如下:AlN-DTDPP2 (-190.78) >;BNPP2 (-185.09) >;CCPP2(-14.86)。极化率的增加表明纳米管对DTD的吸附形成了SERS效应,并且在配合物的拉曼光谱中存在DTD中不存在的振动模式。对于不同的尝试频率,发现恢复时间非常低,对于所有CC-DTD, AlN-DTDPP1和BN-DTDPP3。对于AlN/BN-DTDPP2,恢复时间非常高,并且具有良好的传感效果。对接分数高表明纳米管具有药物载体活性。对在水和甲醇中具有较高吸附能的配合物进行了分子动力学模拟。本文章由计算机程序翻译,如有差异,请以英文原文为准。
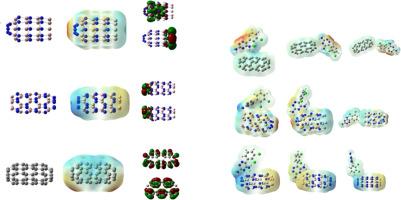
Unlocking the potential of Lamotrigine in nanotubes: DFT, MD simulations in different solvents, sensing properties and drug enhancer
Using density functional theory, the adsorption properties of lamotrigine (6-(2,3-dichlorophenyl)1,2,4-triazine-3,5-diamine) (DTD) with CC, AlN and BN nanotubes are reported. Different configurations are selected for optimization. The study addresses the need for efficient drug carriers by evaluating nanotubes (CC, BN, AlN) for lamotrigine (DTD) delivery. Key findings include: PP2 (NH₂-end) has the highest adsorption energy (–190.78 kJ/mol for AlN); SERS effects confirm DTD-nanotube binding, and MD shows stability in water/methanol. In all cases, DTD at the end of the nanotubes give maximum adsorption energy. For all complexes, adsorption energy varies as AlN-DTDPP2 (-190.78) > BNPP2 (-185.09) > CCPP2 (-14.86). The increase in polarizability suggests SERS effect is formed due to adsorption of DTD with nanotubes and the vibrational modes which are absent in the DTD is present in the Raman spectra of complexes. For different attempt frequencies the recovery times are found and very low for all CC-DTD, AlN-DTDPP1 and BN-DTDPP3. For AlN/BN-DTDPP2, the recovery times are very high and the sensing effects are also presented. High docking scores indicate the drug carrier activity of nanotubes. MD simulations are carried out for the complexes giving higher adsorption energy in water and methanol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


