选择氧化光催化剂NiWO4作为助催化剂提高TiO2/rGO在太阳光下制氢性能的策略
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文采用简单的湿浸渍法制备了TiO2/NiWO4/rGO纳米复合材料。实验和表征结果表明NiWO4 (NW)和rGO在提高TiO2光催化制氢性能方面有显著贡献。虽然NW是一种氧化电位更高的氧化光催化剂,但其与TiO2的合适能带边对齐有利于TiO2/NW与交错II型异质结界面的Z-scheme电子通路。此外,Ni2+离子在TiO2表面晶格中取代Ti3+和Ti4+,促进了单电子氧空位的形成,使光生空穴被困住,增强了电子对H+离子还原的参与。有趣的是,NW的VB中具有高正氧化电位的空穴有利于水分子氧化成H+离子。Z-scheme电子途径促进了TiO2的CB中的激发电子对H+离子的还原而不重组。这些被激发的电子被另一种助催化剂rGO快速传递,具有高电子亲和性和导电性。因此,所合成的纳米复合材料具有较高的界面电荷转移效率(IFCT)和较低的电荷转移阻力。缺陷态能级和C与O重叠的p轨道减小了带隙,增强了可见光吸收。因此,在太阳直射下,纳米复合材料的光催化效率是原始TiO2的3倍以上。这项工作强调了在选择助催化剂以增强纳米复合材料所需的性能以提高其性能方面的深刻见解的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
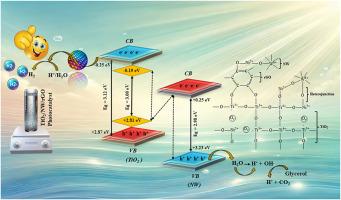
The strategy on selecting the oxidation photocatalyst NiWO4 as a co-catalyst to enhance the performance of TiO2/rGO for H2 production under solar light
In this work, TiO2/NiWO4/rGO nanocomposite was prepared by a simple wet impregnation method. The experimental and characterization results revealed the significant contribution of NiWO4 (NW) and rGO in enhancing the photocatalytic performance of TiO2 for H2 production. Though NW is an oxidation photocatalyst with more positive oxidation potential, its suitable band edge alignment with TiO2 facilitated a Z-scheme electron pathway at the interface of TiO2/NW with the staggered type II heterojunction. Additionally, Ni2+ ions substitution for Ti3+ and Ti4+ in the surface lattice of TiO2 enhanced the formation of single electron oxygen vacancies, which trapped the photogenerated holes and enhanced the electrons’ participation in H+ ions reduction. Interestingly, the holes in the VB of NW with high positive oxidation potential favour the oxidation of water molecules into H+ ions. The Z-scheme electron pathway facilitated the excited electrons in the CB of TiO2 for the H+ ions reduction without recombination. These excited electrons are quickly transported by another co-catalyst rGO, with high electron affinity and conductivity. Hence, the high interfacial charge transfer efficiency (IFCT) with reduced charge transfer resistance was observed for the synthesized nanocomposite material. Defect state energy levels and the overlapped p-orbitals of C and O reduced the band gap and enhanced the visible light absorption. Hence, the nanocomposite showed more than 3 times the photocatalytic efficiency of the pristine TiO2 under direct solar light. This work highlights the necessity for profound insight in selecting the co-catalyst to reinforce the desired properties in a nanocomposite material to improve its performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


