结合MIC13、GRA1和SAG1抗原的BALB/c小鼠刚地弓形虫多表位候选疫苗的设计和免疫学评价
IF 3.1
Q2 PARASITOLOGY
引用次数: 0
摘要
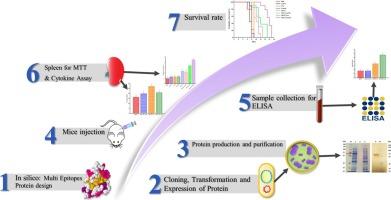
弓形虫病是由顶端复合寄生虫刚地弓形虫引起的,是一种全球重要但被忽视的疾病,可对人类造成严重的临床后果,并给畜牧业造成广泛损失。然而,目前还没有针对弓形虫的有效疫苗,因此本研究旨在评估一种嵌合多表位抗原作为弓形虫病潜在候选疫苗在小鼠模型中的免疫原性。利用生物信息学工具MGS:弓形虫MIC13、GRA1和SAG1抗原嵌合体设计的多表位候选疫苗在大肠杆菌BL21中表达,并通过固定化金属亲和层析纯化。小鼠分别在第0、21和35天用MGS蛋白单独免疫或辅以弗氏佐剂、磷酸钙(CaPNs)或壳聚糖(CNs)纳米佐剂免疫。通过ELISA检测比较MGS(单独或与弗洛伊德氏、CNs或CaPNs联合)与对照组(PBS、弗洛伊德氏、CNs、CaPNs)的体液和细胞免疫应答。MGS蛋白,无论是单独使用还是与佐剂配合使用,都能显著提高特异性抗体滴度,尤其是IgG2a亚型和细胞因子IFN-γ。MGS-Freund组总抗体、IFN-γ和IL-4水平最高。RH型弓形虫攻毒后BALB/c小鼠脾脏淋巴细胞增殖率提高,存活率提高。结果表明,MGS蛋白显著增强了实验组的Th1和Th2免疫应答。这些结果支持多表位疫苗作为开发有效弓形虫病疫苗的一种有希望的策略的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design and immunological evaluation of a multi-epitope vaccine candidate against Toxoplasma gondii incorporating MIC13, GRA1, and SAG1 antigens in BALB/c mice
Toxoplasmosis, caused by the apicomplexan parasite Toxoplasma gondii, is a globally significant yet neglected disease that can cause serious clinical consequences in humans and extensive losses in the livestock industry. However, no effective vaccine has been provided for this parasite, so this study was designed to evaluate the immunogenicity of a chimeric multi-epitope antigen as a potential toxoplasmosis vaccine candidate in a murine model. The multi-epitope vaccine candidate, designed with bioinformatics tools, MGS: a chimera of T. gondii MIC13, GRA1, and SAG1 antigens, was expressed in Escherichia coli BL21 and purified by immobilized metal affinity chromatography using a His Ni-NTA column. Mice were immunized with MGS protein alone or adjuvanted with Freund's adjuvant, calcium phosphate (CaPNs), or chitosan (CNs) nano-adjuvants on days 0, 21, and 35. Humoral and cellular immune responses to MGS (alone or adjuvanted with Freund's, CNs, or CaPNs) were compared to control groups (PBS, Freund's alone, CNs alone, CaPNs alone) through ELISA assays. The MGS protein, either alone or formulated with adjuvants, significantly increased specific antibody titers, particularly the IgG2a subtype and the cytokine IFN-γ. The highest levels of total antibodies, IFN-γ, and IL-4 were observed in the MGS-Freund group. It also enhanced the proliferation rate of splenic lymphocytes and improved the survival rate of BALB/c mice following challenge with the RH strain of Toxoplasma. The findings demonstrate that the MGS protein significantly enhances both Th1 and Th2 immune responses in experimental groups. These results support the efficacy of multi-epitope vaccines as a promising strategy for the development of effective vaccines against toxoplasmosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Waterborne Parasitology
Immunology and Microbiology-Parasitology
CiteScore
5.10
自引率
4.00%
发文量
38
审稿时长
13 weeks
期刊介绍:
Food and Waterborne Parasitology publishes high quality papers containing original research findings, investigative reports, and scientific proceedings on parasites which are transmitted to humans via the consumption of food or water. The relevant parasites include protozoa, nematodes, cestodes and trematodes which are transmitted by food or water and capable of infecting humans. Pertinent food includes products of animal or plant origin which are domestic or wild, and consumed by humans. Animals and plants from both terrestrial and aquatic sources are included, as well as studies related to potable and other types of water which serve to harbor, perpetuate or disseminate food and waterborne parasites. Studies dealing with prevalence, transmission, epidemiology, risk assessment and mitigation, including control measures and test methodologies for parasites in food and water are of particular interest. Evidence of the emergence of such parasites and interactions among domestic animals, wildlife and humans are of interest. The impact of parasites on the health and welfare of humans is viewed as very important and within scope of the journal. Manuscripts with scientifically generated information on associations between food and waterborne parasitic diseases and lifestyle, culture and economies are also welcome. Studies involving animal experiments must meet the International Guiding Principles for Biomedical Research Involving Animals as issued by the Council for International Organizations of Medical Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


