合成美利柯酮A和B的高级中间体的仿生方法
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-22
DOI:10.1021/acs.joc.5c0073410.1021/acs.joc.5c00734
引用次数: 0
摘要
本文描述了一种仿生方法,用于合成合成美利克隆酮A和B的高级中间体,从而成功构建了这些分子的关键双环[3.2.1]辛烷碳框架。该策略的关键步骤包括Wolff重排,钛介导的giese型环化和硝酰化/重排过程。我们的方法能够可靠地组装全功能化三环前体,这反过来又为进一步细化目标分子提供了有价值的处理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
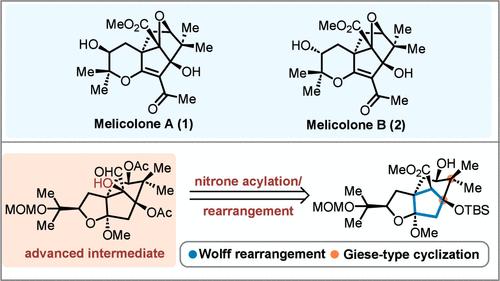
A Biomimetic Approach for Construction of an Advanced Intermediate en Route to Melicolones A and B
A biomimetic approach for the synthesis of an advanced intermediate en route to melicolones A and B is described, leading to successful construction of the crucial bicyclo[3.2.1]octane carbon framework of these molecules. Key steps of the strategy include a Wolff rearrangement, a titanium-mediated Giese-type cyclization, and a nitrone acylation/rearrangement process. Our approach enables the reliable assembly of a fully functionalized tricyclic precursor, which in turn provides valuable handles for further elaboration of the target molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


