含螺胍环戊烷的C(sp3) -H氧化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-26
DOI:10.1021/acs.joc.5c0071410.1021/acs.joc.5c00714
引用次数: 0
摘要
建立了5元环螺胍类化合物的C(sp3) -H氧化反应。在三氟乙醇中使用h2o2 -尿素引入环戊烷-融合的氨基甲基吡啶作为环胍部分酰胺键的导向基团,使环戊烷环的螺旋β-位置发生氧化。这允许在螺旋β位置选择性地引入羟基,并适用于具有各种取代基的环戊烷化合物,包括那些难以用普通氧化方法功能化的化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
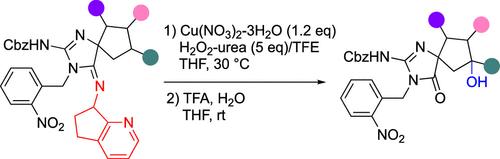
C(sp3)–H Oxidation of Spiroguanidine-Containing Cyclopentanes
A C(sp3)–H oxidation reaction for spiroguanidine compounds with 5-membered rings was developed. The introduction of a cyclopentane-fused aminomethylpyridine as a directing group to the amide linkage of the cyclic guanidine moiety, using H2O2–urea in trifluoroethanol, enabled oxidation at the spiro β-position of the cyclopentane ring. This allowed selective introduction of a hydroxy group at the spiro β-position and was applicable to cyclopentane compounds with various substituents, including those challenging to functionalize with common oxidation methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


