2h -吲哚的电化学区域选择性C-3烷氧羰基化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-23
DOI:10.1021/acs.joc.5c0078510.1021/acs.joc.5c00785
引用次数: 0
摘要
本研究描述了一种不需要外部氧化剂或金属催化剂的情况下,在不分裂电池中恒流条件下,与氨基甲酸酯进行2h -吲哚的C-3烷氧羰基化的高效电化学合成方法。该方法具有广泛的官能团相容性,产品收率高。易于扩展和药物多样化证明了该方案的合成有效性和适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
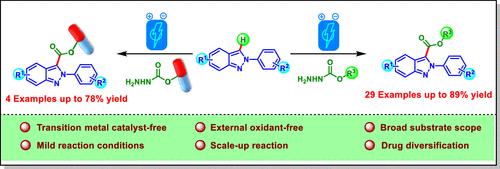
Electrochemical Regioselective C-3 Alkoxycarbonylation of 2H-Indazoles
This study describes an efficient electrochemical synthetic method for C-3 alkoxycarbonylation of 2H-indazoles with carbazates under constant current in an undivided cell without the need for external oxidants or metal catalysts. This methodology exhibits broad functional group compatibility, affording the products with good yields. Easy scalability and drug diversification demonstrate the synthetic efficacy and applicability of this protocol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


