dabco催化的亲核芳香取代及其在改进作为CLIP-Tag底物的o2 -苄基胞嘧啶合成中的应用
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们描述了1,4-重氮杂环[2.2.2]辛烷(DABCO),一种亲核叔胺,在合成o2 -苄基胞嘧啶的反应效率和操作方便性方面的影响。苄胞嘧啶是蛋白质标记工具CLIP-tag的底物。介绍了合成的CLIP-tag底物在哺乳动物活细胞质膜荧光标记中的应用。为了更全面地描述DABCO在含氮杂环的亲核芳香取代(SNAr)反应中的作用,研究了DABCO催化的氯化嘧啶取代的效率和范围。用单晶x射线衍射和核磁共振光谱对嘧啶单、双dabco加合物的结构进行了表征。对DABCO加合物的SNAr反应性进行了评价。讨论了DABCO在SNAr反应中作为亲核催化剂的优点和局限性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
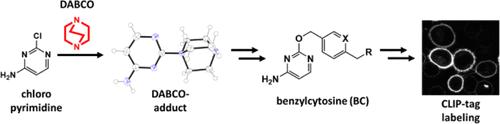
DABCO-Catalyzed Nucleophilic Aromatic Substitution and Its Use in Improving the Synthesis of O2-Benzylcytosines as Substrates of CLIP-Tag
We describe the effect of 1,4-diazabicyclo[2.2.2]octane (DABCO), a nucleophilic tertiary amine, in the synthesis of O2-benzylcytosines in terms of both reaction efficiency and operational convenience. Benzylcytosines are substrates of the protein-labeling tool CLIP-tag. The applications of the synthesized CLIP-tag substrates in the fluorescent labeling of plasma membranes of live mammalian cells are demonstrated. For providing a fuller depiction of the function of DABCO in nucleophilic aromatic substitution (SNAr) reactions involving nitrogen heterocycles, the efficiency and scope of DABCO-catalyzed substitutions on chlorinated pyrimidines are studied. The structures of single- and double-DABCO adducts of pyrimidine are characterized by single-crystal X-ray diffraction and NMR spectroscopy. The SNAr reactivities of the DABCO adducts are assessed. Both the benefits and limitations of DABCO as a nucleophilic catalyst in SNAr reactions are discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


