含混合电解质盐电解质在锂离子电池电化学储能装置中的应用与进展
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
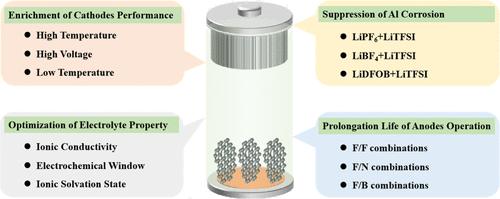
电解质盐作为一种不可缺少的组成部分,对电化学储能装置的性能有着巨大的影响。然而,每种电解质盐都不能满足可充电电池对电解质的所有需求。例如,在锂离子电池中,诸如六氟磷酸锂(LiPF6)基电解质在高温下分解、二氟磺酰胺锂(LiFSI)或双三氟甲烷磺酰亚胺锂(LiTFSI)基电解质的Al阴极集流器腐蚀、四氟硼酸锂(LiBF4)基电解质的低导电性等缺点,二氟草酸锂(LiDFOB)或二草酸锂(LiBOB)基电解质不稳定的电化学窗口限制了可充电电池单一锂盐基电解质的进一步发展。在这种情况下,人们花了很多精力来克服每种电解质盐固有的不足,其中混合电解质盐的策略越来越受到人们的关注。本文综述了混合电解质盐的研究进展,包括混合电解质盐对电解质性能优化的影响、Al集流器的性能、各种阴极、下一代阳极和充满电池的性能。这篇综述旨在阐明混合电解质盐在如何影响电池组件中的作用,最终改变各种可充电电池的性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Utilization and Advancement of an Electrolyte Containing Mixed Electrolyte Salts in Electrochemical Energy Storage Devices Mainly Based on Lithium-Ion Batteries
An electrolyte salt as an indispensable component has a dramatic impact on the performance of electrochemical energy storage devices. However, every electrolyte salt cannot satisfy all the needs of an electrolyte for rechargeable batteries. For example, in lithium-ion batteries, shortages like the decomposition of the lithium hexafluorophosphate (LiPF6)-based electrolyte at high temperature, the corrosion to the Al cathodic current collector of the lithium difluorosulfonamide (LiFSI)- or lithium bistrifluoromethanesulfonylimide (LiTFSI)-based electrolyte, the low conductivity of the lithium tetrafluoroborate (LiBF4)-based electrolyte, and the inappropriately stable electrochemical windows of the lithium difluorooxalateborate (LiDFOB)- or lithium bisoxalatoborate (LiBOB)-based electrolyte restrict further development of the single lithium salt-based electrolyte for the rechargeable batteries. In this case, much effort has been spent in overcoming the inherent shortage of each electrolyte salt, in which the strategy of mixing an electrolyte salt is attracting growing attention. Herein, an overview about the research progress surrounding the mixed electrolyte salts is presented, including the effect of mixed electrolyte salts on the property’s optimization of the electrolyte, the performance of the Al current collector, various cathodes, next-generation anodes, and full batteries. This review aims to elucidate the role of a mixed electrolyte salt in how it influences the battery’s components, ultimately changing the performance of various rechargeable batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


