晶体面增强了欠配位Pt1-TiO2单原子物种的甲烷氧化动力学稳定性和反应活性
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在实际反应过程中,载体的晶面对单原子催化剂动态演化的影响尚不明确。在此,我们报道了晶体面介导的Pt1-TiO2 SACs的甲烷氧化动力学演化和反应性。在反应条件下,TiO2(001)有利于低配位Ptδ+物质(0 <;δ& lt;2)与Ti3+-Ov基团(Ptδ+-Ov-Ti3+)结合具有较高的活性,而TiO2(101)表面稳定的Pt4+与饱和的Pt-O配位几乎没有活性。载体的晶面和反应环境对反应过程中Ptδ+-Ov-Ti3+的自发形成至关重要,表现出不同于其热还原稳定性的独特动态稳定性。Ptδ+-Ov-Ti3+酸性增强,有利于C-H活化和O2解离,有利于甲烷氧化。本研究阐明了Ptδ+-Ov-Ti3+在反应过程中晶面增强的稳定性和反应活性,为基于其动态稳定性的SACs的合理设计开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
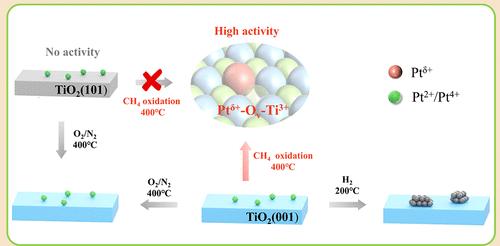
Crystal Facet Enhanced Dynamic Stability and Reactivity of Undercoordinated Pt1-TiO2 Single Atom Species for Methane Oxidation
The influence of crystal facets of the support on the dynamic evolution of single-atom catalysts (SACs) during real reactions remains elusive. Herein, we report a crystal facet mediated dynamic evolution and reactivity of Pt1-TiO2 SACs for methane oxidation. Under reaction conditions, the TiO2(001) favored dynamic formation of undercoordinated Ptδ+ species (0 < δ < 2) in association with Ti3+-Ov moieties (Ptδ+-Ov-Ti3+) for high activity, whereas TiO2(101) surface stabilized Pt4+ species with saturated Pt–O coordination showing nearly no activity. Both the crystal facet of the support and the reaction environment are crucial for spontaneous formation of Ptδ+-Ov-Ti3+ species during reaction, showing unique dynamic stability that is different from their thermal/reduction stability. The enhanced acidity of Ptδ+-Ov-Ti3+ facilitates C–H activation and O2 dissociation for methane oxidation. This work elucidates crystal facet enhanced stability and reactivity of Ptδ+-Ov-Ti3+ species during reaction and paves a new route for the rational design of SACs based on their dynamic stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


