可见光诱导的不饱和醛腙自由基级联二氟烷基化/环化合成二氟烷基化二氢吡唑和四氢吡嗪
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在可见光诱导下,n -烯丙基和均烯丙基醛腙与各种氟烷基溴化物发生了5/6-内三羟基二氟烷基化/环化反应。以二氟乙酸烷基酯和二氟乙酰胺为二氟烷基化试剂,以伊红Y为有机光催化剂进行反应。在温和的无金属条件下,制备了多种二氟烷基化的二氢吡唑和四氢吡嗪。本文章由计算机程序翻译,如有差异,请以英文原文为准。
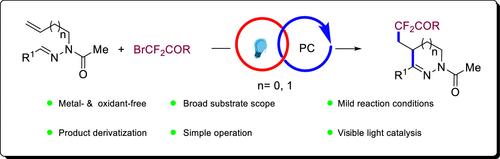
Visible Light-Induced Radical-Cascade Difluoroalkylation/Cyclization of Unsaturated Aldehyde Hydrazones for the Synthesis of Difluoroalkylated Dihydropyrazoles and Tetrahydropyridazines
Visible light-induced radical 5/6-endo-trig difluoroalkylation/cyclization of N-allyl and homoallyl aldehyde hydrazones with various fluoroalkyl bromides has been achieved. The reaction was carried out with alkyl difluoroacetates and difluoroacetamide as difluoroalkylation reagents and Eosin Y as an organic photocatalyst. Various difluoroalkylated dihydropyrazoles and tetrahydropyridazines were obtained under metal-free and mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


