细胞内金属纳米粒子和离子的差异定位和动态建模预测
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在生物医学领域,预测金属纳米颗粒(MNPs)的毒性仍然是一个长期存在的挑战,主要是由于在生命系统中,原始MNPs与其溶解离子对抗物之间尚未解决的动态转化。在此,我们开发了一个综合的生物成像数学框架,以实时模式量化MNPs及其离子对应物对毒性的贡献。通过集成基于聚集诱导发射(AIE)的共聚焦成像和无标记散射光跟踪,我们实现了不同尺寸的原始银、氧化铜和氧化锌纳米颗粒(Ag-、CuO-和ZnO-NPs, 20-100 nm)及其在活细胞中的离子形态的同步和无创可视化。这种双模态方法揭示了细胞内溶解动力学的大小依赖性,2.68-34.7%的内化MNPs在摄取后溶解,小颗粒释放的离子是大颗粒的1.08-1.22倍。利用这些时空洞察力,我们开发了一个级联毒性模型,将细胞外溶解、细胞摄取、细胞内转化和毒性途径机械地联系起来。该模型表明,在0-100 mg/L范围内,离子种类在所有MNPs中占主导地位,占总毒性的59.7-79.4% (AgNPs), 69.6-100% (CuO-NPs)和97.7% (ZnO-NPs)。引人注目的是,毒性特征因MNP类型而异:AgNPs表现出双相毒性,CuO-NPs遵循类似物流的模式,而ZnO-NPs完全是离子驱动的。通过将实时生物成像与动力学建模相结合,我们的框架提供了纳米颗粒与离子特异性毒性的第一个体内定量分辨率。这项工作不仅促进了对MNP行为的机制理解,而且还建立了一种普遍适用的预测纳米毒理学工具,使纳米材料的设计更安全,并为监管政策提供了信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
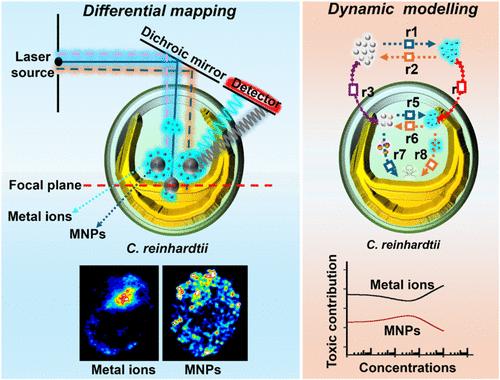
Differential Mapping of Intracellular Metallic Nanoparticles and Ions and Dynamic Modeling Prediction
Predicting the toxicity of metallic nanoparticles (MNPs) remains a longstanding challenge in the biomedical field, primarily due to the unresolved dynamic transformation between pristine MNPs and their dissolved ionic counterparts within living systems. Herein, we develop an integrative bioimaging-mathematical framework that quantifies, in real-time mode, the contributions of MNPs and their ionic counterparts to toxicity. By integrating aggregation-induced emission (AIE)-based confocal imaging with label-free scattered light tracking, we achieve simultaneous and noninvasive visualization of different-sized pristine silver, copper oxide, and zinc oxide nanoparticles (Ag-, CuO-, and ZnO-NPs, 20–100 nm) and their ionic forms in living cells. This dual-modal approach reveals size-dependent intracellular dissolution dynamics, with 2.68–34.7% of internalized MNPs dissolving post uptake and smaller particles releasing 1.08–1.22 times more ions than larger particles. Leveraging these spatiotemporal insights, we developed a cascading toxicity model that mechanistically links extracellular dissolution, cellular uptake, intracellular transformation, and toxicity pathways. The model demonstrates that ionic species dominate toxicity across all MNPs, contributing 59.7–79.4% (AgNPs), 69.6–100% (CuO-NPs), and 97.7% (ZnO-NPs) of overall toxicity within 0–100 mg/L. Strikingly, toxicity profiles vary by MNP type: AgNPs exhibit biphasic toxicity, CuO-NPs follow a logistic-like pattern, and ZnO-NPs remain entirely ion-driven. By bridging real-time bioimaging with kinetic modeling, our framework provides the first in vivo quantitative resolution of nanoparticle- versus ion-specific toxicity. This work not only advances mechanistic understanding of MNP behavior but also establishes a universally applicable tool for predictive nanotoxicology, enabling safer design of nanomaterials and informed regulatory policies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


