结肠保真度的丧失使多系可塑性和转移成为可能
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
癌细胞的可塑性能够获得新的表型特征,并被认为是转移进展的主要驱动因素1,2。转移大多发生在没有额外遗传改变的情况下,这表明表观遗传机制很重要。然而,它们的定义仍然很模糊。在这里,我们发现染色质重塑酶ATRX是结直肠癌结肠谱系保真度和转移的关键调节因子。基质缺失促进肿瘤侵袭和转移,同时伴有结肠上皮特性的丧失和高度可塑性的间充质和鳞状细胞状态的出现。对染色质可及性和增强子定位的联合分析发现,结肠谱系特异性转录因子HNF4A的活性受损是这些观察到的表型的关键介质。我们在人类患者样本中鉴定出鳞状样细胞和鳞状样表达特征,这些特征与侵袭性疾病和不良患者预后相关。综上所述,我们的研究确定了ATRX和HNF4A对结肠上皮特性的表观遗传维持是结直肠癌谱系可塑性和转移的抑制因子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
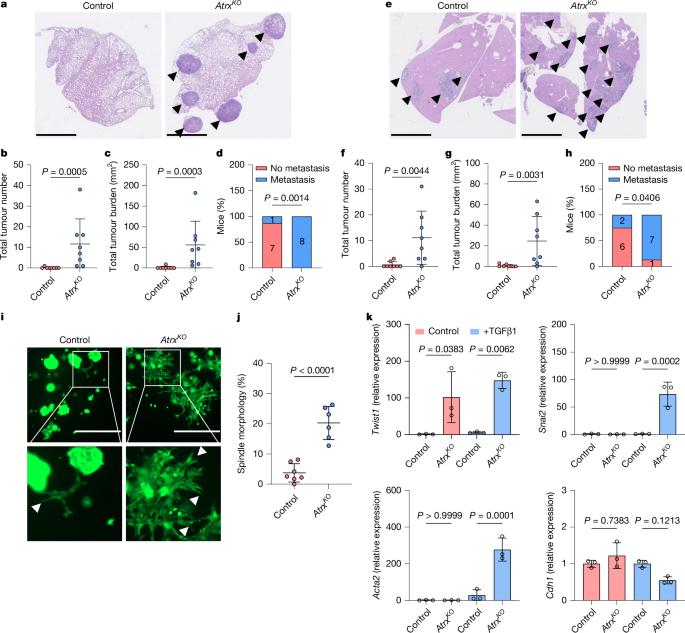
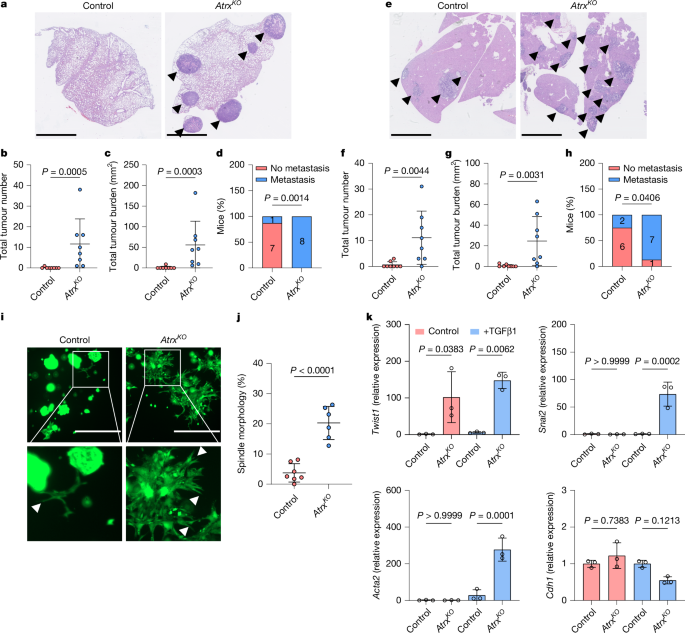
Loss of colonic fidelity enables multilineage plasticity and metastasis
Cancer cell plasticity enables the acquisition of new phenotypic features and is implicated as a major driver of metastatic progression1,2. Metastasis occurs mostly in the absence of additional genetic alterations3–5, which suggests that epigenetic mechanisms are important6. However, they remain poorly defined. Here we identify the chromatin-remodelling enzyme ATRX as a key regulator of colonic lineage fidelity and metastasis in colorectal cancer. Atrx loss promotes tumour invasion and metastasis, concomitant with a loss of colonic epithelial identity and the emergence of highly plastic mesenchymal and squamous-like cell states. Combined analysis of chromatin accessibility and enhancer mapping identified impairment of activity of the colonic lineage-specifying transcription factor HNF4A as a key mediator of these observed phenotypes. We identify squamous-like cells in human patient samples and a squamous-like expression signature that correlates with aggressive disease and poor patient prognosis. Collectively, our study defines the epigenetic maintenance of colonic epithelial identity by ATRX and HNF4A as suppressors of lineage plasticity and metastasis in colorectal cancer. The chromatin-remodelling enzyme ATRX and the transcription factor HNF4A are identified as pivotal regulators of colonic epithelial identity, with roles in metastasis in colorectal cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


