pH和温度对聚乳酸水解动力学的影响
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
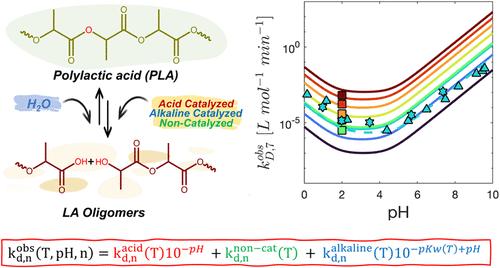
本研究考察了不同操作条件下聚乳酸(PLA)的水解降解,以确定聚合物水解分解的微动力学。实验研究考虑了温度、pH、聚乳酸初始摩尔质量等因素的影响。采用高效液相色谱法(HPLC)跟踪试剂和降解产物的浓度随时间的变化。建立了一个综合随机链断裂、优先端链断裂和背咬反应的动力学模型。动力学参数是通过拟合实验数据得到的,得到了每个反应的速率常数作为链长、温度和ph的函数的广义表达式。具体地说,反应速率分为三个不同的贡献:碱催化、非催化和酸催化机制。最终,这种功能形式在几种基于乳酸的大分子中得到验证,强调了它对聚酯和聚酰胺的适用性。由此产生的机制模型具有强大的预测性,可作为设计和优化堆肥过程的有价值的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Influence of pH and Temperature on the Kinetics of Polylactic Acid Hydrolysis
This study investigates the hydrolytic degradation of polylactic acid (PLA) under different operating conditions to define the microkinetics of polymer decomposition through hydrolysis. The experimental study accounts for the effect of various factors such as temperature, pH, and initial molar mass of the PLA analyzed. High-performance liquid chromatography (HPLC) was adopted to track the concentrations of reagents and degradation products over time. A comprehensive kinetic model was developed, which integrated random chain scission, preferential end-chain scission, and backbiting reactions. The kinetic parameters were derived by fitting experimental data, yielding a generalized expression for the observed rate constant of each reaction as a function of chain length, temperature, and pH. Specifically, the reaction rates were broken down into three distinct contributions: alkaline-catalyzed, uncatalyzed, and acid-catalyzed mechanisms. Ultimately, this functional form is validated across several lactic-acid–based macromolecules, underscoring its applicability to polyesters and polyamides. The resulting mechanistic model exhibits robust predictivity, serving as a valuable tool for the design and optimization of composting processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


