选择性电催化硝酸还原为二氮:机制、催化剂和挑战
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
摘要
电催化还原硝酸盐为二氮(N2)提供了一种可持续的、碳中性的方法,通过将硝酸盐转化为对环境无害的最终产品来减轻硝酸盐污染。虽然广泛的研究集中在氨的生产上,但由于复杂的途径、检测的局限性以及在电催化剂上控制反应的难度,选择性生产N2仍然具有挑战性。本文综述了该领域的最新进展,重点介绍了机理、N2检测方法和催化剂设计的进展。各种类型的电催化剂,包括单金属、双金属和单原子系统、氧化物基和碳基材料,研究它们的结构如何调节关键中间体和神经网络耦合。同时对应用电位、电解液、pH值和反应器设计等操作参数的影响进行了综述。尽管取得了重大进展,但在理解界面机制和在现实条件下实现高N2选择性方面仍然存在挑战。本文旨在为未来高效电催化系统的发展提供及时的见解和指导,以实现硝酸盐选择性转化为n2,推进环境修复和可持续的氮管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
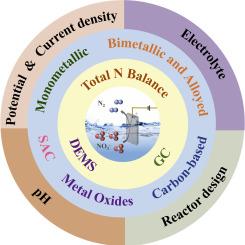
Selective electrocatalytic nitrate reduction to dinitrogen: Mechanisms, catalysts, and challenges
Electrocatalytic reduction of nitrate to dinitrogen (N2) offers a sustainable, carbon-neutral approach to mitigate nitrate pollution by transforming nitrate into an environmentally benign end-product. While extensive research has focused on ammonia production, selective N2 production remains challenging due to complex pathways, limitations in detection, and the difficulty of controlling reaction on the electrocatalysts. This review provides an overview of current progress in this field, focusing on mechanistic insights, N2 detection methods, and advances in catalyst design. Various classes of electrocatalysts, including monometallic, bimetallic, and single-atom systems, oxide-based and carbon-based materials, examining how their structures regulate key intermediates and N![]() N coupling. The influence of operational parameters such as applied potential, electrolyte, pH, and reactor design is also reviewed. Despite significant advances, challenges remain in understanding interfacial mechanisms and achieving high N2 selectivity under realistic conditions. This review aims to offer timely insights and guidance for the future development of efficient electrocatalytic systems toward selective nitrate-to-N2 conversion, advancing environmental remediation and sustainable nitrogen management.
N coupling. The influence of operational parameters such as applied potential, electrolyte, pH, and reactor design is also reviewed. Despite significant advances, challenges remain in understanding interfacial mechanisms and achieving high N2 selectivity under realistic conditions. This review aims to offer timely insights and guidance for the future development of efficient electrocatalytic systems toward selective nitrate-to-N2 conversion, advancing environmental remediation and sustainable nitrogen management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


