具有平行排列棒状纳米晶的分级t型沸石的简易合成
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
通过调节SiO2/Al2O3的摩尔比和结晶动力学,简单地合成了具有平行排列棒状纳米晶体(宽约73 nm)的分级t型沸石(HT沸石)。选择面积电子衍射(SAED)分析表明,纳米棒的晶体生长方向沿c轴,而短轴沿a和b方向。优化后的高温沸石骨架Si/Al比为2.74,BET表面积高达489.6 m2/g。合成凝胶中SiO2/Al2O3的摩尔比严重影响了沸石的结构、粒径和形貌,形成了具有较低8摩尔比的核桃状CHA相。高温沸石在373 K下加热48 h,得到高结晶性的高温沸石,其结晶曲线呈s形,其中棒状纳米晶体以平行排列的方式生长,同时保持几乎恒定的宽度。为了研究其成核和晶体生长行为,进一步进行了不同温度下的Gualtieri模型分析和时间跟踪实验。在353 ~ 403 K的温度范围内,根据Arrhenius关系式计算成核活化能和晶体生长活化能分别为43.0±3.2和36.5±1.7 kJ mol−1,表明成核是限制速率的步骤。高温沸石在1 bar和273 K条件下的CO2吸附量为4.01 mol/kg, CO2/N2初始和最终吸附比分别为40.4和5.4。本文提出了一种简单有效的制备分级T沸石的方法,可为其他分级材料的设计提供参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
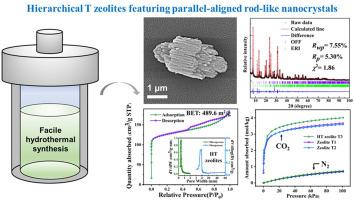
Facile synthesis of hierarchical T-type zeolites with parallel-aligned rod-like nanocrystals
Hierarchical T-type zeolites (HT zeolites) with parallel-aligned rod-like nanocrystals (∼73 nm in width) were synthesized via a facile method simply by regulating the SiO2/Al2O3 molar ratio and crystallization kinetics. Selected area electron diffraction (SAED) analysis revealed that the crystallographic growth direction of the nanorods was along the c-axis while the short axis was along a- and b-directions. The optimized HT zeolites exhibited a framework Si/Al ratio of 2.74 and a high BET surface area (489.6 m2/g). The SiO2/Al2O3 molar ratio in the synthesis gel critically influenced zeolite structure, particle size, and morphology, inducing a competing CHA phase with a walnut-shaped morphology with lower ratio of 8. Highly crystalline HT zeolites were obtained at 373 K after 48 h, following an S-shaped crystallization curve, where rod-like nanocrystals grew in a parallel alignment while maintaining nearly constant widths. To investigate the nucleation and crystal growth behavior, Gualtieri model analysis and time trace experiments at different temperature were further conducted. The activation energies for nucleation and crystal growth, calculated from the Arrhenius relationship in the temperature range of 353–403 K, were found to be 43.0 ± 3.2 and 36.5 ± 1.7 kJ mol−1 respectively, indicating nucleation was the rate-limiting step. The HT zeolites exhibited a CO2 adsorption capacity of 4.01 mol/kg at 1 bar and 273 K, with CO2/N2 initial and final adsorption ratios of 40.4 and 5.4, respectively. This work presents a simple and effective method for preparing hierarchical T zeolites, which can serve as a possible reference for the design of other hierarchical materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


