双氯芬酸钠和文拉法辛在微塑料上的吸附行为:基于光谱和密度泛函理论的机理研究
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
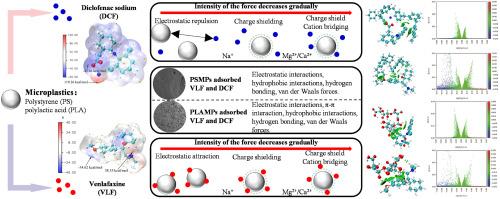
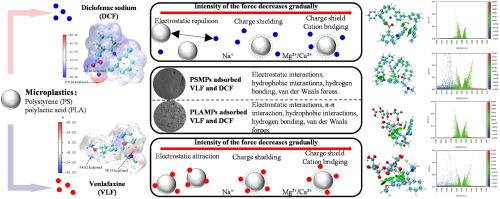
双氯芬酸钠(DCF)和文拉法辛(VLF)是广泛使用的环境持久性药物污染物,在水体中经常被检测到,并表现出生物积累和生态毒性,可能通过破坏水生生物的内分泌系统和神经信号来威胁生态系统的稳定性。这些污染物与微塑料(MPs)的结合不仅增强了污染物的迁移,而且放大了通过食物链暴露于高营养级生物的风险,这对于研究全球“塑料圈”中水生环境的安全性和污染物耦合机制具有重要的警示意义。本研究探讨了DCF和VLF在聚苯乙烯(PSMPs)和聚乳酸(PLAMPs)两种不同MPs上的吸附行为和机理。批量吸附实验用于定量控制溶液化学条件下的吸附能力,检查吸附动力学,等温线和热力学。PLAMPs和PSMPs对DCF的最大吸附量分别为2.994和3.296 mg g-1, PLAMPs和PSMPs对VLF的最大吸附量分别为5.864和6.234 mg g-1。DCF和VLF在两种MPs上的吸附主要依赖于物理相互作用,动力学模型与拟一阶模型一致,等温线与Langmuir模型一致。傅里叶红外光谱和x射线光电子能谱显示了吸附前后MPs表面官能团的变化。两种MPs在吸附DCF和VLF后,氧基和氮基官能团均显著增加,变化最明显的是C-O和C=O的增加。利用密度泛函理论分析了PhACs和MPs的主要吸附机理和结合能。综上所述,我们的研究发现DCF和VLF在MPs上的吸附是由静电相互作用控制的,其次是一些较弱的相互作用(即范德华力、氢键和疏水相互作用)。该研究为复杂污染物之间有效分离和相互作用的机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sorption behavior of diclofenac sodium and venlafaxine on microplastics: a mechanistic study based on spectroscopy and density functional theory
Diclofenac sodium (DCF) and venlafaxine (VLF), as widely used and environmentally persistent pharmaceutical pollutants, are frequently detected in water bodies and exhibit bioaccumulation and ecotoxicity, which may threaten the stability of ecosystems by disrupting the endocrine systems of aquatic organisms and neural signaling. The combination of these pollutants with microplastics (MPs) not only enhances the migration of the pollutants, but also amplifies the risk of exposure to high trophic level organisms via the food chain, which is an important warning significance for the study of the safety of the aquatic environment and the mechanism of pollutant coupling in the global “plastic circle”. This study delved into the sorption behavior and mechanisms of DCF and VLF on two different MPs (i.e., polystyrene (PSMPs) and polylactic acid (PLAMPs)). Batch sorption experiments were used to quantify sorption capacity under controlled solution chemistry conditions, examining sorption kinetics, isotherms, and thermodynamics. The maximum sorption capacity of PLAMPs and PSMPs for DCF was 2.994 and 3.296 mg g−1, respectively, while that of PLAMPs and PSMPs for VLF was 5.864 and 6.234 mg g−1, respectively. The sorption of DCF and VLF on both MPs mainly depended on physical interactions, with the kinetic model in agreement with the pseudo-first-order model, the isotherms were consistent with the Langmuir model. Fourier infrared spectroscopy and X-ray photoelectron spectroscopy demonstrated variations regarding surface functional groups upon MPs before and after sorption. Both MPs showed a significant increase in oxygen and nitrogen-based functional groups after sorption of DCF and VLF, with the most obvious variations being the increase in C-O and C=O. Density-functional theory was used to detect the main sorption mechanisms and the binding energies between PhACs and MPs. In summary, our study found the sorption of DCF and VLF on MPs is controlled by electrostatic interactions, followed by some weaker interactions (i.e., van der Waals forces, hydrogen bonding, and hydrophobic interactions). This study provides new insights into the mechanisms of effective separation and interaction between complex pollutants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


