hfc -152基包合物水合物用于合成采出水脱盐的相行为、动力学和能源效率
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
笼形水合物脱盐(CHD)是一项新兴的技术,它通过生成水合物晶体来分离含盐水中的盐分,从而产生淡水。本研究以含盐11.94 wt %的合成采出水(SPW)为水合物前体,探讨了HFC-152a作为CHD的潜力。相平衡和动力学分析表明,盐起到抑制剂的作用,将水合物的形成转移到更高的压力和更低的温度,同时延长诱导时间,减少气体的吸收。能源分析表明,总能耗(TEC)主要由HFC-152a压缩和制冷驱动,在40%的水回收率下,比能耗(SEC)为104.77 kWh/m3。实施HFC-152a回收后,TEC降至42.86 kW, SEC降至79.34 kWh/m3。敏感性分析证实了该工艺在不同盐度下的稳定性。这些发现强调了CHD作为一种有前途的、节能的海水淡化替代方案。未来的研究应集中在优化参数和解决大规模应用的扩展挑战上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
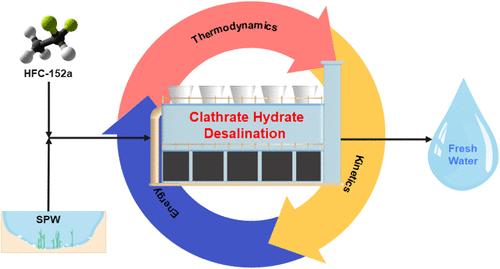
Phase Behavior, Kinetics, and Energy Efficiency with Recycling of HFC-152a-Based Clathrate Hydrate Desalination for Synthetic Produced Water
Clathrate hydrate desalination (CHD) is an emerging technology that generates hydrate crystals to separate salts from saline water, producing fresh water. This study explores the potential of CHD using HFC-152a as the hydrate former with synthetic produced water (SPW) containing 11.94 wt % salts. Phase equilibrium and kinetic analyses revealed that salts act as inhibitors, shifting hydrate formation to higher pressures and lower temperatures while prolonging induction times and reducing gas uptake. Energy analysis showed that total energy consumption (TEC) was primarily driven by HFC-152a compression and refrigeration, with a specific energy consumption (SEC) of 104.77 kWh/m3 at a 40% water recovery rate. Implementing HFC-152a recycling reduced TEC to 42.86 kW and SEC to 79.34 kWh/m3. Sensitivity analysis confirmed the process stability across salinities. These findings highlight CHD as a promising, energy-efficient desalination alternative. Future research should focus on optimizing parameters and addressing scaling challenges for large-scale applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


