压缩表面活性剂单层的自发曲率诱导毛细压力
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
通过分子动力学模拟,我们发现,随着表面活性剂在汽液界面上的覆盖范围的增加,表面活性剂单层形成,然后压缩,导致表面张力发生三个阶段的变化。表面活性剂达到饱和吸附后,不溶性表面活性剂覆盖面积的进一步增加会对单层膜进行压缩,从而使单层膜刚度降低,不仅使表面张力降至超低值,而且会影响相邻相之间的力学平衡。模拟结果表明,虽然与表面活性剂单层的界面大致平坦,但共存相的平衡压力可能被打破。模拟结果和热力学分析表明,与传统的由界面曲率引起的毛细压力不同,本文所确定的新型毛细压力(压差)是由压缩表面活性剂单层的非零自发曲率引起的。这种非零自发曲率诱导的毛细压力也表现出尺寸效应,即毛细压力随单层尺寸的增大而减小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
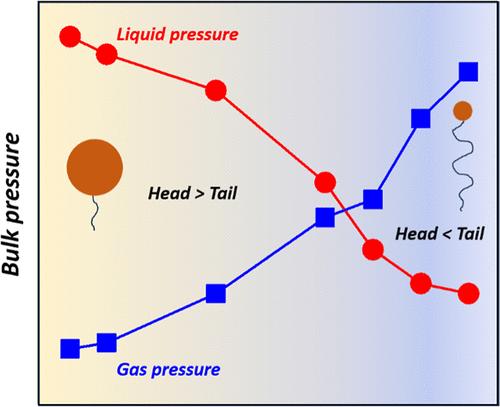
Spontaneous Curvature of Compressed Surfactant Monolayer Induces Capillary Pressure
Using molecular dynamics simulations, we show that as surfactant coverage at a vapor–liquid interface increases, a surfactant monolayer forms and then compresses, causing a three-stage change in the surface tension. After saturated surfactant adsorption is reached, a further increase in the coverage of insoluble surfactant would compress the monolayer, and the resulting monolayer rigidity would not only lower the surface tension to ultralow value but also impact the mechanical equilibrium between neighboring phases. The simulation results indicate that the equilibrium pressure of coexisting phases may break down, although the interface with the compressed surfactant monolayer is roughly flat. The simulation results along with the thermodynamic analysis reveal that, different from the conventional capillary pressure that is induced by the interface curvature, the new type of capillary pressure (pressure difference) identified here is caused by the nonzero spontaneous curvature of the compressed surfactant monolayer. This nonzero spontaneous curvature-induced capillary pressure also shows a size effect, i.e., the capillary pressure decreases with the increase of monolayer size.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


