烯胺类硫铵盐的重排和环化:取代吲哚的有效获取。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-06-13
Epub Date: 2025-06-02
DOI:10.1021/acs.joc.5c00299
引用次数: 0
摘要
公开了一种用于构建吲哚化合物的易碱介导的烯胺噻吩盐的环化和重排。在这个反应中,两个C-S键的连续断裂和随后形成的C-N和C-C键提供了一系列的取代吲哚,收率中等到较高。此外,建立了一个高效的后期功能化的烯胺化合物。该方法的特点是衬底范围广,官能团耐受性好,起始材料成本低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
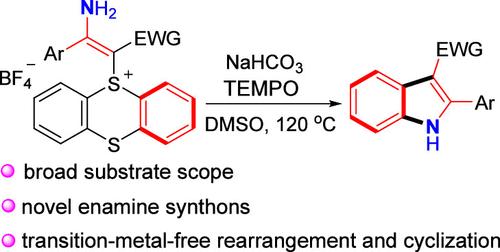
Rearrangement and Cyclization of Enamine Thianthrenium Salts: Effective Access to Substituted Indoles.
A facile base-mediated cyclization and rearrangement of enamine thianthrenium salts for the construction of indole compounds is disclosed. In this reaction, sequential cleavage of two C-S bonds and subsequent formation of C-N and C-C bonds furnish a series of substituted indoles in moderate to good yields. Furthermore, an efficient late-stage functionalization of enamine compounds is established. The method features a wide substrate scope, good functional group tolerance, and low-cost starting materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


